Stoichiometry Made Simple: Grams to Grams Conversion Answers

The journey into chemistry often begins with understanding how substances react, how much of them react, and how much they produce. One of the fundamental aspects of this study is stoichiometry, particularly converting from grams to grams in chemical reactions. This process not only unravels the mystery of chemical quantities but also ensures precision in experiments and practical applications.
Understanding Stoichiometry
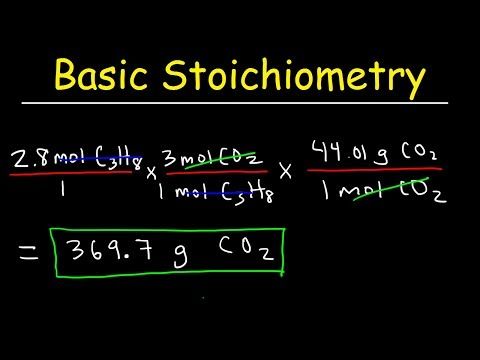
Stoichiometry is essentially the mathematics of chemistry. It involves calculating the quantitative relationships between reactants and products in a chemical reaction. Here's how you can grasp the concept:
- Balanced Chemical Equations: The foundation of stoichiometry. They tell you the exact molar ratios in which compounds react.
- Molar Ratios: Use these ratios to convert from moles of one substance to moles of another.
- Mole-to-Mole Conversions: Before converting grams to grams, we often first convert grams to moles, then moles to moles, and finally moles back to grams.
Let's delve into how grams-to-grams conversion works in practice.
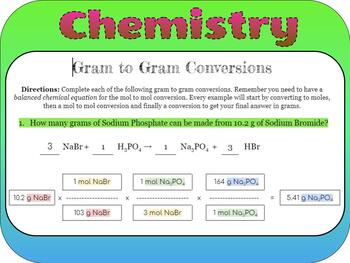
Steps for Grams to Grams Conversion

Here are the steps you need to follow:
- Write and Balance the Chemical Equation: This equation gives you the stoichiometric relationship between reactants and products.
- Calculate Molar Mass: For each compound involved, calculate its molar mass by adding up the atomic masses from the periodic table.
- Convert Grams to Moles: Use the molar mass to convert the grams of your starting substance into moles.
- Use Molar Ratios: Apply the coefficients from the balanced equation as the ratio for moles conversion.
- Convert Moles to Grams: Use the molar mass of the product to convert back to grams.
Let's illustrate with an example:
Example: Ammonium Nitrate Decomposition
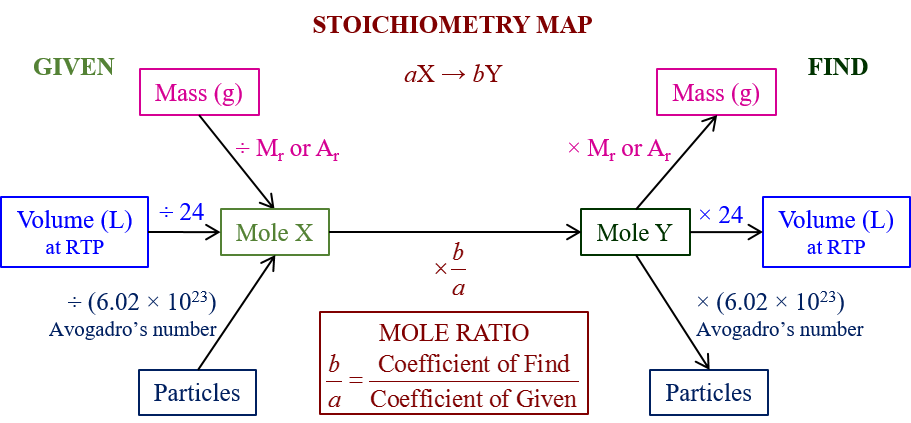
Consider the decomposition of ammonium nitrate (NH₄NO₃) to form dinitrogen monoxide (N₂O) and water vapor (H₂O):
NH₄NO₃(s) → N₂O(g) + 2H₂O(g)
If we start with 100g of NH₄NO₃, here’s how we would convert:
| Step | Process | Calculation |
| 1 | Calculate Molar Mass of NH₄NO₃ | 14.01 (N) + 1.01 × 4 (H) + 14.01 (N) + 16.00 × 3 (O) = 80.05 g/mol |
| 2 | Convert grams to moles | 100 g / 80.05 g/mol = 1.249 moles of NH₄NO₃ |
| 3 | Use Molar Ratio from Balanced Equation | 1 mole of NH₄NO₃ yields 1 mole of N₂O |
| 4 | Convert Moles to Grams for N₂O | 1.249 moles × 44.02 g/mol (N₂O’s molar mass) = 55.0 g |
💡 Note: Ensure you always use the correct molar masses from your periodic table to maintain accuracy in calculations.
Common Pitfalls in Stoichiometry Calculations

- Ignoring Significant Figures: Always consider significant figures when reporting your answer to maintain precision.
- Miscalculating Molar Masses: Misunderstanding atomic mass units or rounding incorrectly can lead to errors.
- Failing to Balance the Equation: An unbalanced equation will result in incorrect molar ratios.
⚠️ Note: A common mistake in stoichiometry is not considering the stoichiometry of the reaction. The ratio of coefficients matters.
Practical Applications of Stoichiometry

Stoichiometry isn't just an academic exercise; it has real-world implications:
- Industrial Production: Companies use stoichiometry to determine raw material needs and product yields.
- Environmental Impact: Chemical engineers use stoichiometry to minimize waste and emissions in manufacturing.
- Pharmacology: Drug synthesis relies on precise stoichiometric calculations to ensure correct dosing and effective compounds.
In wrapping up, we've covered the essence of stoichiometry, particularly grams-to-grams conversions. Through balanced equations, understanding molar masses, and following a methodical approach, we can predict chemical reactions with high accuracy. This not only enhances our theoretical knowledge but also has practical applications in everyday life, from pharmaceutical development to industrial processes. Remembering the key steps and avoiding common pitfalls will ensure you're equipped to tackle any stoichiometry problem with confidence.
Why is stoichiometry important in chemistry?

+
Stoichiometry helps us understand how much reactant is required to produce a given amount of product, enabling precise chemical measurements for research, industry, and academic learning.
Can stoichiometry predict the outcome of a reaction?

+
Yes, stoichiometry can predict the amounts of products formed if you know the quantities of reactants and have a balanced chemical equation.
What if the reaction isn’t balanced? How do you fix that?

+
If the reaction isn’t balanced, you must adjust the coefficients of reactants and products until the number of atoms of each element is the same on both sides of the equation.