Unlock Chemistry Success: Intermolecular Forces Worksheet Solutions

Intermolecular forces (IMFs) play a crucial role in understanding the physical and chemical properties of substances. Mastery of these forces is not only vital for acing your chemistry exams but also for unlocking the deeper understanding required to excel in advanced sciences. This blog post is designed to provide detailed solutions to common intermolecular forces worksheet questions, enhancing your learning experience and helping you grasp the underlying principles of IMFs.
Understanding Intermolecular Forces
Before diving into the solutions, let's quickly review the types of intermolecular forces:
- Ionic Bonds: These occur between ions, involving a complete transfer of electrons.
- Hydrogen Bonding: A specialized form of dipole-dipole interaction where hydrogen is bonded to fluorine, nitrogen, or oxygen.
- Dipole-Dipole Forces: These arise between polar molecules, where partial charges attract each other.
- London Dispersion Forces (LDF): Also known as van der Waals forces, these temporary fluctuations in electron distribution create momentary dipoles.
Worksheet Solutions: Intermolecular Forces
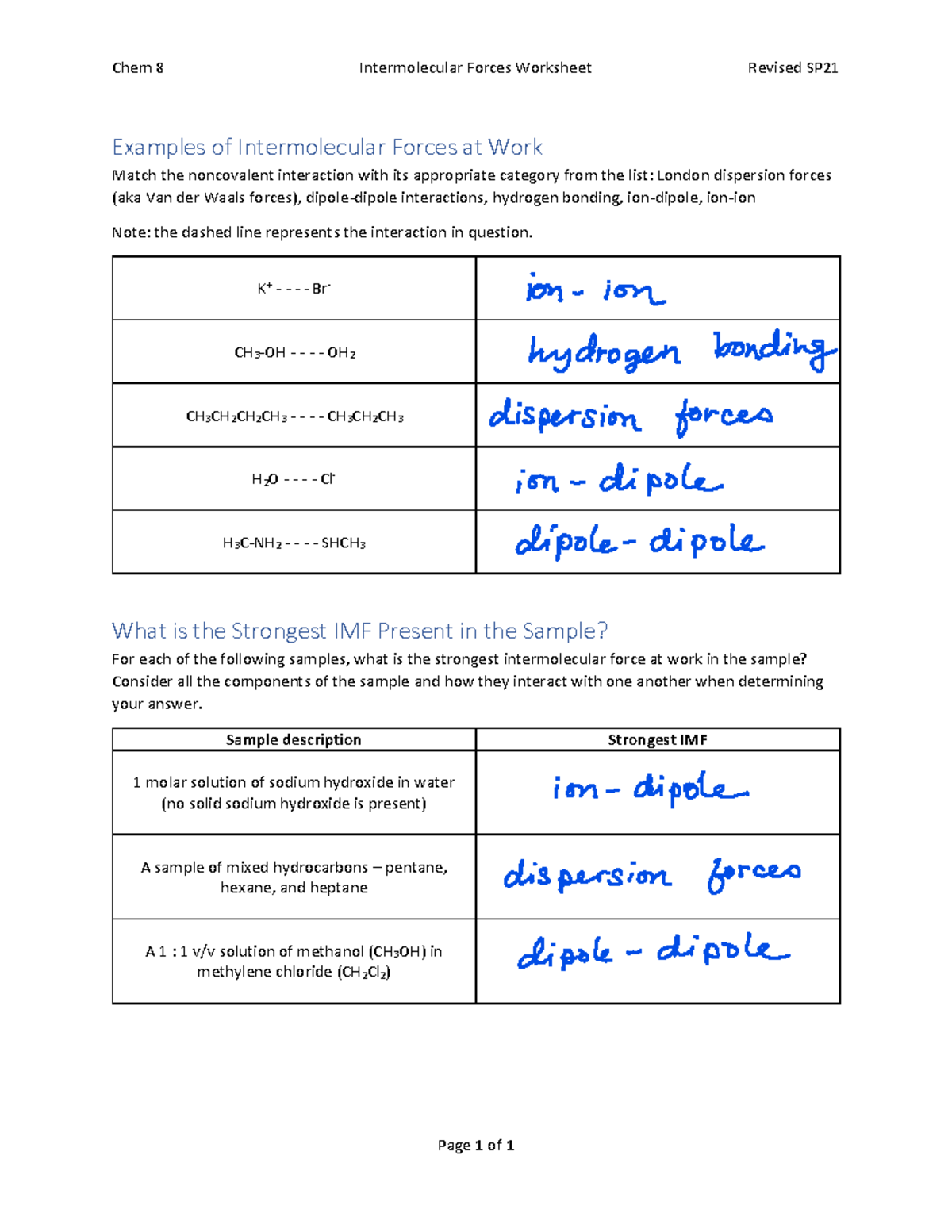
Question 1: Identify the Predominant IMF in Given Molecules

Given the following molecules, identify the predominant intermolecular force:
- HCl
- CH4
- NH3
| Molecule | Predominant IMF |
|---|---|
| HCl | Dipole-Dipole Forces |
| CH4 | London Dispersion Forces |
| NH3 | Hydrogen Bonding |

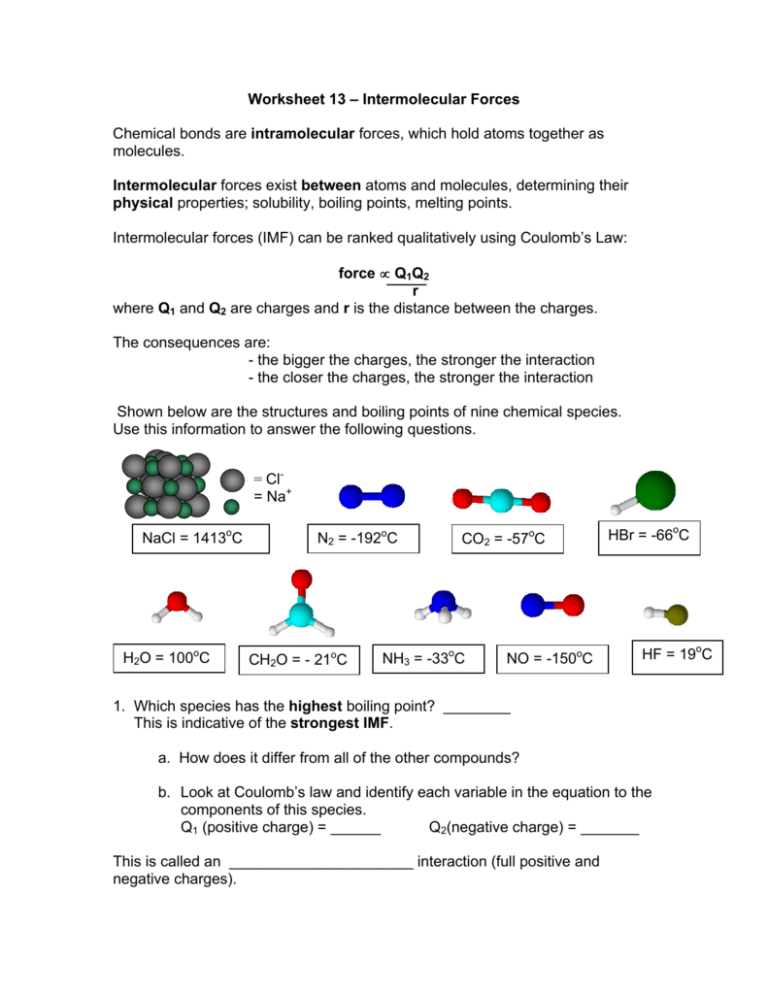
Question 2: Rank the Following Compounds in Order of Boiling Points

Consider the following compounds:
- CH3CH2CH2CH3
- CH3OH
- H2O
- CH3OCH3
Rank them from lowest to highest boiling points:
🔍 Note: Higher boiling points are associated with stronger intermolecular forces. Thus, we need to consider hydrogen bonding, molecular size, and polarity.
- Lowest boiling point: CH3OCH3 (Only LDF due to its non-polar nature)
- Next: CH3CH2CH2CH3 (LDF with larger molecule size)
- Next: CH3OH (Hydrogen bonding but weaker than water)
- Highest: H2O (Strong hydrogen bonding due to its small size and strong electronegativity of oxygen)
Question 3: Surface Tension and Intermolecular Forces

Explain why mercury has a high surface tension, even though it is a metal.
- Mercury has metallic bonding, which results in very strong attractions between its atoms.
- Its surface tension arises from the strong cohesive forces within the liquid that minimize surface area.
- Despite being a metal, mercury's intermolecular forces (metallic bonds) are very strong, leading to its high surface tension.
Question 4: Solubility and Intermolecular Forces
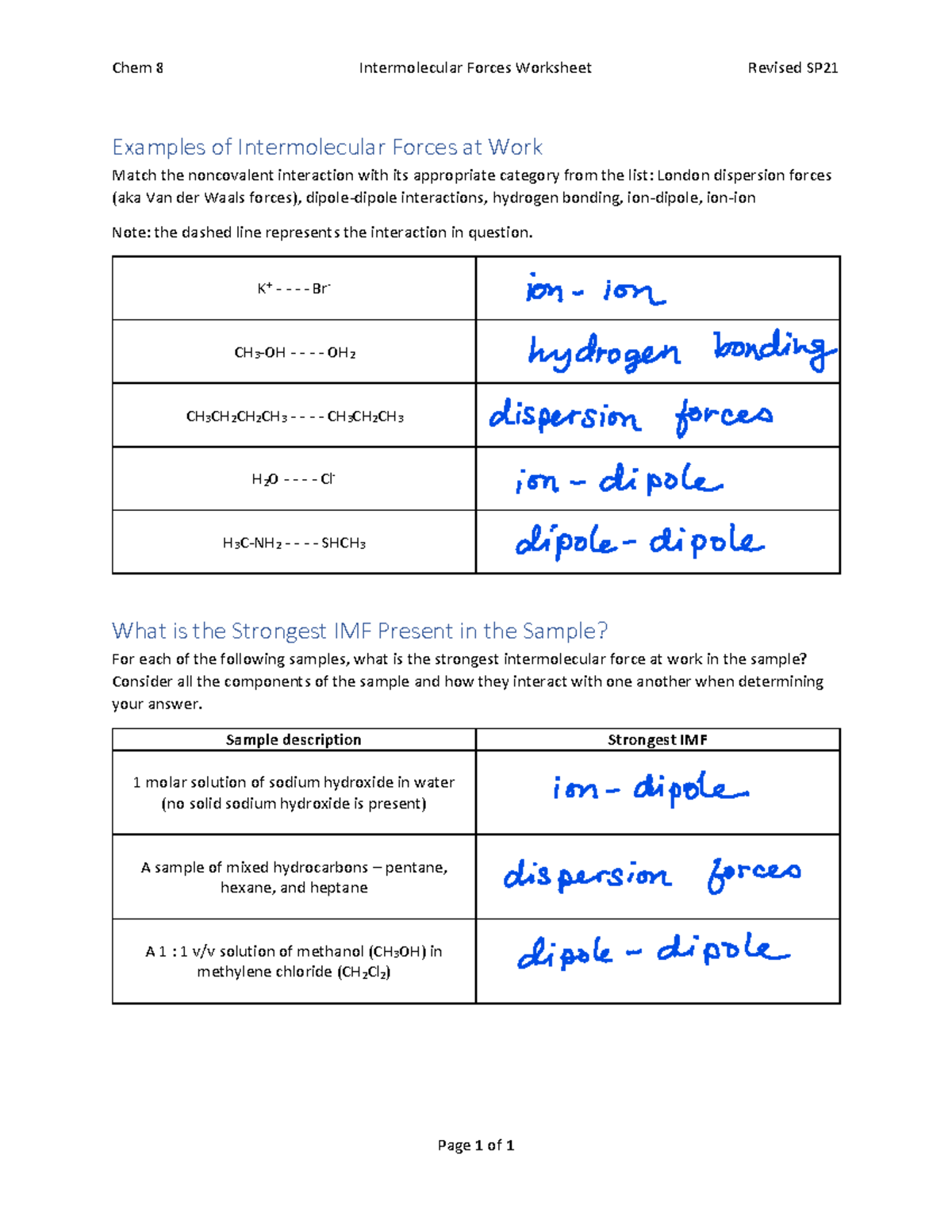
Predict whether or not each of these substances will dissolve in water:
- C6H14 (hexane)
- CH3OH
- NaCl
Hexane (C6H14): Non-polar, will not dissolve in water due to the "like dissolves like" principle.
Methanol (CH3OH): Polar, can form hydrogen bonds with water; will dissolve.
Sodium Chloride (NaCl): Ionic compound, will dissociate into ions, forming ion-dipole interactions with water; will dissolve.
Question 5: Viscosity and Intermolecular Forces

Why does glycerol have a much higher viscosity than water?
Glycerol (C3H5(OH)3) has:
- Three hydroxyl groups, allowing it to form more hydrogen bonds than water.
- These hydrogen bonds result in stronger intermolecular forces, leading to higher viscosity.
In summary, this guide has walked you through various scenarios involving intermolecular forces. By understanding these forces, you can better predict physical properties like boiling points, solubility, and viscosity, which are not only crucial in theoretical studies but also in practical applications in various industries.
What are intermolecular forces?

+
Intermolecular forces are the forces that act between molecules. They include ionic, hydrogen bonding, dipole-dipole, and London dispersion forces.
How do intermolecular forces affect boiling points?

+
Stronger intermolecular forces require more energy (heat) to break the molecular bonds, thus increasing the boiling point of a substance.
Why is surface tension high in mercury?

+
Mercury’s high surface tension is due to the strong metallic bonds between its atoms, which create significant cohesive forces within the liquid.