7 Key Differences: Intensive vs. Extensive Properties

The physical and chemical properties of substances play a crucial role in understanding their behavior under different conditions. Two fundamental concepts in the study of physical chemistry are intensive and extensive properties. These terms help us categorize properties in a way that aids scientific understanding and practical applications. Here are the seven key differences between intensive and extensive properties, illustrated in detail:
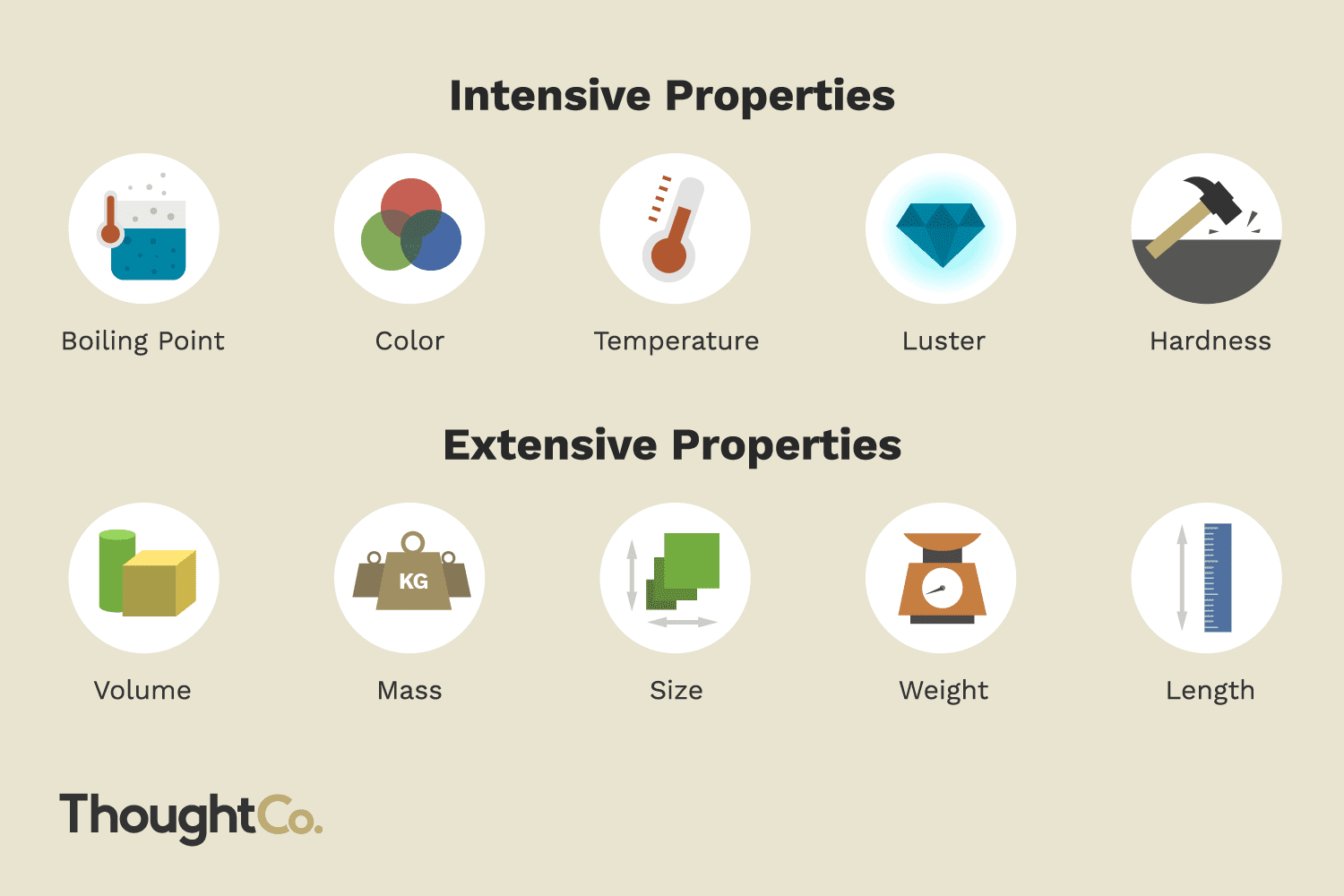
1. Definition

Intensive Properties: These are properties that do not depend on the quantity of matter in a system. They are independent of the size or amount of the sample. Examples include:
- Density
- Pressure
- Temperature
- Concentration (molarity, molality)
- Refractive Index
- Color
Extensive Properties: Conversely, these properties do depend on the quantity of matter present in the system. They increase or decrease as the system's size changes. Examples include:
- Volume
- Mass
- Energy (internal energy, enthalpy)
- Amount of Substance (moles)
💡 Note: A common way to remember these definitions is: "Intensive properties are intense – they're about the nature, not the amount."
2. Measurement

Intensive Properties: To measure these, you only need to consider a portion or a very small sample of the substance:
- Measure the temperature of any point in the substance to know its temperature everywhere.
Extensive Properties: These require measuring the entire substance:
- Measure the entire volume or mass of the substance.
3. Additivity

Intensive Properties: These properties are not additive. If you divide a substance into smaller parts:
- Each part will have the same intensive property as the whole.
Extensive Properties: They are additive. When you combine systems:
- The total extensive property will be the sum of individual values from each part.
4. Application in Chemistry

Intensive Properties:
- Useful in phase diagrams where changes in intensive properties like pressure and temperature cause phase transitions without altering the substance's amount.
- They're essential for understanding intrinsic qualities of substances.
Extensive Properties:
- Critical for calculating quantities, stoichiometry, and energy balances in chemical reactions.
- They aid in designing equipment or containers that must hold or process a particular amount of substance.
5. Examples in Practice
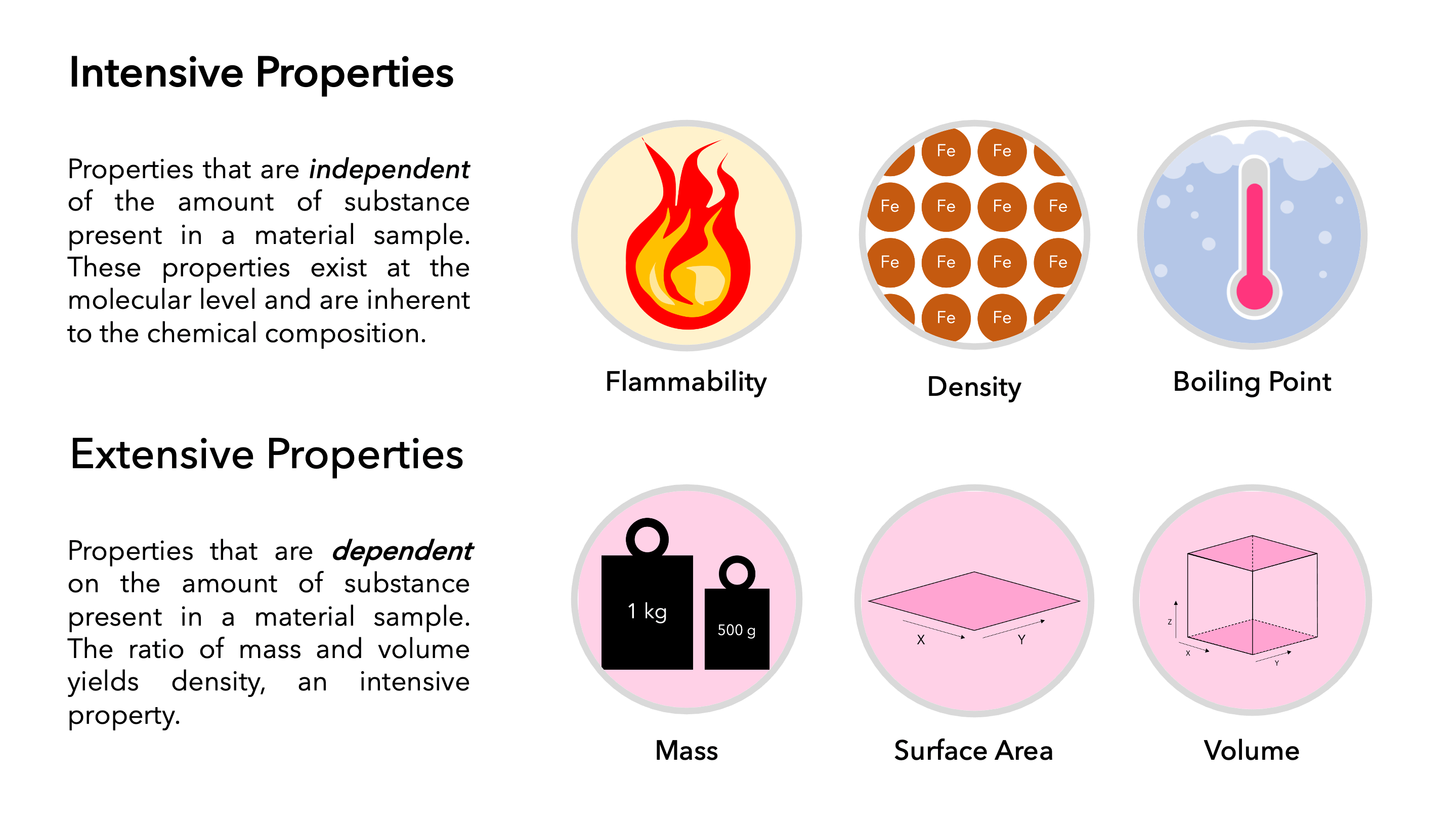
Intensive Properties: Here's how they apply in real-world scenarios:
| Intensive Property | Example |
|---|---|
| Density | A 100 ml sample of water has the same density as a 1 L sample of water. |
| Boiling Point | Whether you boil 1 ml or 1000 ml of water, it boils at 100°C at standard atmospheric pressure. |

Extensive Properties: These are crucial in understanding bulk quantities:
| Extensive Property | Example |
|---|---|
| Mass | The mass of water in a bucket is the sum of the mass of each molecule within it. |
| Volume | The volume of air in a balloon increases as more air is pumped into it. |
6. Physical and Chemical Implications

Intensive Properties:
- They define the character of substances, providing insight into their molecular or atomic structure.
- Changes in intensive properties usually indicate phase changes or changes in the state of matter.
Extensive Properties:
- They describe how much of the substance is present, which is essential for mass and energy balance in chemical reactions.
7. Transformation Under Scaling

When considering the behavior of a system under scaling:
Intensive Properties:
- Remain the same when scaling a sample up or down.
Extensive Properties:
- Change proportionally with the scale of the sample. For example, doubling the amount of a substance will double its mass or volume.
📌 Note: Understanding how properties scale with system size can be critical for designing experiments and interpreting results.
As we delve into the distinctions between intensive and extensive properties, it becomes evident how they guide the interpretation of various chemical and physical phenomena. Intensive properties are about the identity or characteristics of substances, unaffected by quantity, while extensive properties tell us about the quantity or extent of the material. This fundamental differentiation not only helps in understanding the behavior of matter but also in the practical application of chemistry and physics in industrial, academic, and everyday scenarios. The correct categorization of properties allows for better predictive modeling, analysis, and the efficient use of resources in science and engineering.
Why is it important to distinguish between intensive and extensive properties?
+
Distinguishing between intensive and extensive properties is crucial for accurate scientific interpretation, experimental design, and data analysis. It helps in understanding how substances behave under different conditions, whether the quantity or the quality of the substance is important.
Can an extensive property become intensive under certain conditions?
+
Technically, no. However, by defining a new property relative to the size of the system, you can sometimes transform an extensive property into an intensive one. For example, specific volume (volume per unit mass) is an intensive property derived from the extensive property of volume.
Are intensive properties always uniformly distributed in a substance?

+
In most cases, yes. For instance, temperature is uniform throughout an ideal gas at equilibrium. However, in real systems, gradients can exist (e.g., temperature gradients in a poorly mixed liquid).