3 Easy Steps to Find Protons, Neutrons, Electrons

Understanding the fundamentals of atomic structure is crucial in the realm of chemistry and physics. Every atom consists of protons, neutrons, and electrons, which give it unique properties. By knowing how to determine the number of each particle, one can gain insights into the behavior of elements in chemical reactions and more. Here are the three straightforward steps to find the protons, neutrons, and electrons in an atom:
Step 1: Identify the Atomic Number and Mass Number

The atomic number (Z) and mass number (A) are crucial in understanding the composition of an atom:
- Atomic Number (Z): This is the number of protons in the nucleus of an atom. You can find it on the Periodic Table of Elements, where each element is assigned a unique atomic number.
- Mass Number (A): This is the sum of protons and neutrons in the nucleus. Often, this is close to the atomic mass but can differ due to isotopic variations.
Here’s how to find these numbers:
- Look up the element on the Periodic Table. The atomic number is listed above the symbol, and the atomic mass (which is close to the mass number) is listed below.
- If given a specific isotope, the mass number will be directly stated. If not, you might have to estimate based on the atomic mass.

⚙️ Note: Atomic mass on the periodic table is the weighted average of isotopes. It can slightly differ from the mass number of a specific isotope.
Step 2: Calculate Neutrons and Electrons

Once you have the atomic number and mass number, calculating neutrons and electrons is straightforward:
- Neutrons: Subtract the atomic number (Z) from the mass number (A) to find the number of neutrons. This formula can be expressed as: Number of Neutrons = Mass Number - Atomic Number.
- Electrons: Normally, in a neutral atom, the number of electrons equals the number of protons. However, ions can have a different electron count. Here’s how you determine it:
- If the ion has a positive charge, subtract that number of electrons from the atomic number.
- If the ion has a negative charge, add that number of electrons to the atomic number.

⚠️ Note: For ions, the total charge on the atom must be considered when calculating electrons.
Step 3: Double-Check with Examples

To ensure you’ve understood the process correctly, let’s apply these steps to some examples:
| Element | Atomic Number (Z) | Mass Number (A) | Protons | Neutrons | Electrons (Neutral Atom) | Electrons (Ion, if applicable) |
|---|---|---|---|---|---|---|
| Carbon (C) | 6 | 12 | 6 | 6 | 6 | - |
| Oxygen (O) | 8 | 16 | 8 | 8 | 8 | - |
| Nitrogen (N) | 7 | 14 | 7 | 7 | 7 | - |

From these examples:
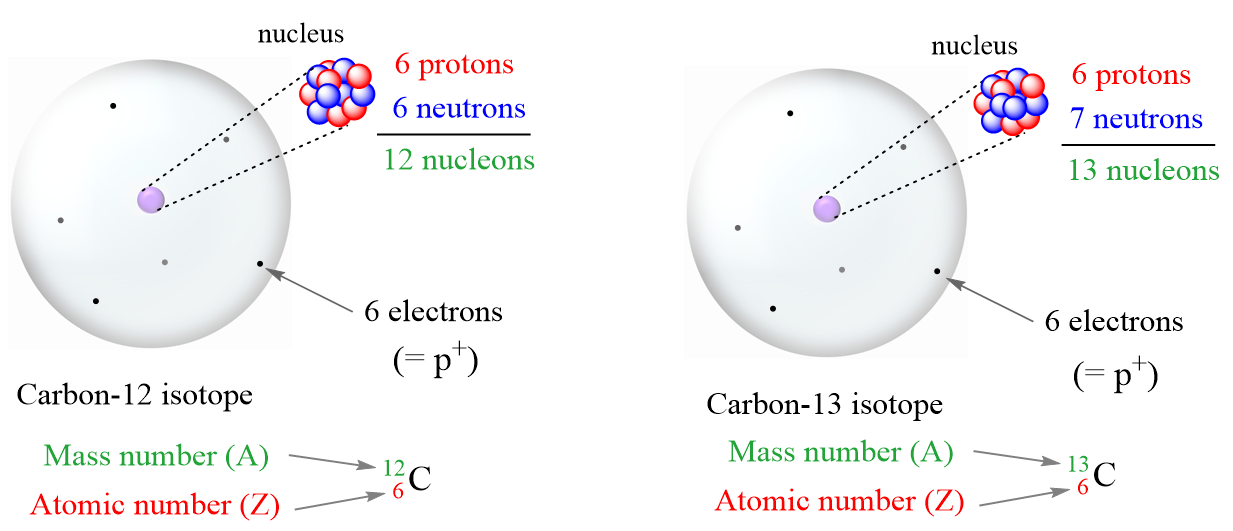
- Carbon: A Carbon-12 (mass number 12) atom has 6 protons, 6 neutrons, and 6 electrons.
- Oxygen: A standard oxygen atom has 8 protons, 8 neutrons, and 8 electrons.
- Nitrogen: A Nitrogen-14 (mass number 14) atom has 7 protons, 7 neutrons, and 7 electrons.
These steps provide a systematic approach to determining the subatomic particles of an atom. Understanding these fundamentals not only helps in academic studies but also in practical applications like nuclear energy, medical diagnostics, and environmental science. With this knowledge, you can now explore and predict how atoms interact, form bonds, and participate in chemical reactions. It’s the beginning of a deeper appreciation for the microscopic world that shapes our macroscopic reality.
What is an ion?

+
An ion is an atom or molecule that has a net electrical charge due to the loss or gain of electrons.
Can atoms have the same number of neutrons?

+
Yes, isotopes of the same element have the same number of protons but different numbers of neutrons.
Why does the number of electrons in an ion differ?
+
In ions, the number of electrons changes to either gain or lose electrons to achieve a more stable electronic configuration or to balance the ion’s charge.
How can I tell the mass number from the atomic mass?

+
The mass number is typically the rounded whole number closest to the atomic mass on the periodic table for the most common isotope of an element.