5 Simple Steps to Ace Balancing Chemistry Equations

Balancing chemical equations can often seem like a daunting task, particularly for those new to chemistry. However, with a systematic approach, anyone can master this skill, turning it into a simple and logical process. This article will guide you through 5 Simple Steps to Ace Balancing Chemistry Equations, ensuring that even beginners can achieve success in this fundamental aspect of chemistry.
Understanding Chemical Equations


Before diving into the steps, it’s crucial to understand what a chemical equation represents. A chemical equation is a symbolic representation of a chemical reaction where reactants (left side) transform into products (right side). The equation must always be balanced to obey the Law of Conservation of Mass, which states that matter can neither be created nor destroyed in a chemical reaction. Here’s what you need to remember:
- Reactants: The substances that react.
- Products: The substances produced by the reaction.
- Coefficients: Numbers placed in front of the chemical formulas to balance the equation.
Step 1: Write Down the Unbalanced Equation

Begin with the chemical reaction you want to balance. Here’s an example:
2 H2 + O2 → 2 H2O
This is an equation for the formation of water from hydrogen and oxygen. Note that this equation is already balanced, but we use it as an example for the process.
Step 2: Count the Atoms on Both Sides

Now, count the number of atoms for each element on the reactant and product sides:
| Element | Reactant Side | Product Side |
|---|---|---|
| H | 4 | 4 |
| O | 2 | 2 |

Make sure you count each element separately and use coefficients if necessary to balance.
Step 3: Start Balancing with the Most Complex Molecule

Typically, you would start with the compound that has the largest number of atoms or the one with the most complex formula. If it’s a combustion reaction, you’d balance the hydrocarbons first.
🔍 Note: For ionic equations, balance polyatomic ions as single units first, if they appear unchanged on both sides.
Step 4: Adjust Coefficients to Balance

Add coefficients in front of the chemical formulas to balance each element:
- Begin with elements that appear in one compound on each side.
- If possible, leave hydrogen and oxygen for last as they often need fractional adjustments.
If you need to balance the oxygen or hydrogen atoms, adjust their coefficients as a fraction if needed, and then multiply the entire equation by the smallest common denominator to get rid of fractions.
Step 5: Check Your Work
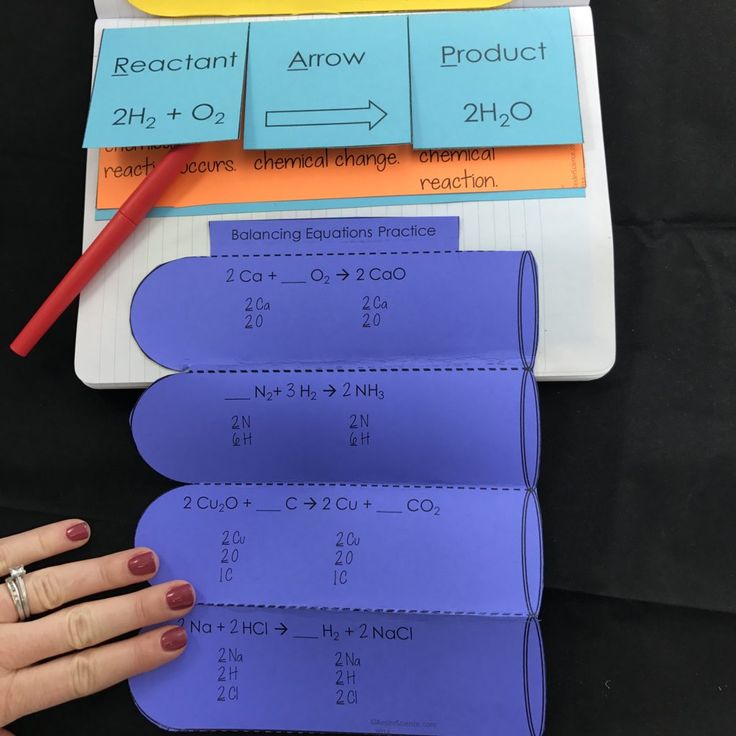
Ensure you’ve balanced all elements correctly:
- Re-count the atoms for each element on both sides.
- If any element is still unbalanced, go back to Step 3 and adjust accordingly.
Lastly, simplify the equation if possible by reducing coefficients. A balanced equation should have the smallest whole numbers as coefficients.
In mastering the art of balancing chemical equations, consistent practice and understanding of the underlying principles are key. By following these 5 Simple Steps to Ace Balancing Chemistry Equations, even the most complex equations become manageable. Remember, each step is designed to logically approach the problem, ensuring every element's mass is conserved through the reaction. With time, this process will become second nature, enhancing your ability to tackle chemical reactions with confidence.
Why must chemical equations be balanced?

+
Chemical equations must be balanced to adhere to the Law of Conservation of Mass. This law states that in a closed system, mass is neither created nor destroyed during a chemical reaction, hence the total mass of the reactants should equal the total mass of the products.
What should I do if I get stuck balancing an equation?

+
If you get stuck, go back to the step where you made your last change or consider another molecule or compound to start with. Sometimes, re-counting the atoms or starting with a different element can reveal the correct approach. Also, ensure you’re not overcomplicating the equation by adding unnecessary coefficients or introducing new elements.
How do I know if my balanced equation is correct?

+
Check that each type of atom is present in equal numbers on both sides of the equation. If you’ve used fractions or large numbers for coefficients, you can simplify the equation by multiplying all coefficients by the smallest common multiple to achieve smaller whole numbers. Then, ensure the chemical symbols and formulas are written correctly.