5 Essential Facts about Atom History Unveiled

Atom’s Existence in Antiquity

Atoms, the fundamental building blocks of matter, have a rich history that traces back to antiquity. As early as 5th century BCE, the concept of atoms was floated by ancient Greek philosophers like Leucippus and his student Democritus. These early philosophers hypothesized that matter consisted of indivisible particles, which they termed “atomos,” meaning uncuttable or indivisible in Greek. They argued that different substances existed because these indivisible particles varied in shape, size, and arrangement. While these theories were purely philosophical, they laid a crucial groundwork for the atomic theory that would evolve over centuries.
Dalton’s Atomic Theory

Fast forward to the early 19th century, British scientist John Dalton is credited with reviving atomic theory through experimental evidence. Here are key points from Dalton’s atomic theory:
- Elements are made of tiny particles called atoms.
- All atoms of a given element are identical in mass and properties.
- Compounds are formed by combining atoms of different elements in fixed, small, whole-number ratios.
- Chemical reactions involve rearrangement, combination, or separation of atoms without creating, destroying, or transmuting them.
🔍 Note: Dalton’s theory was based on earlier work by Lavoisier, Proust, and others, but he was the first to formalize these ideas into a comprehensive theory.
Advancements by Thomson and Rutherford
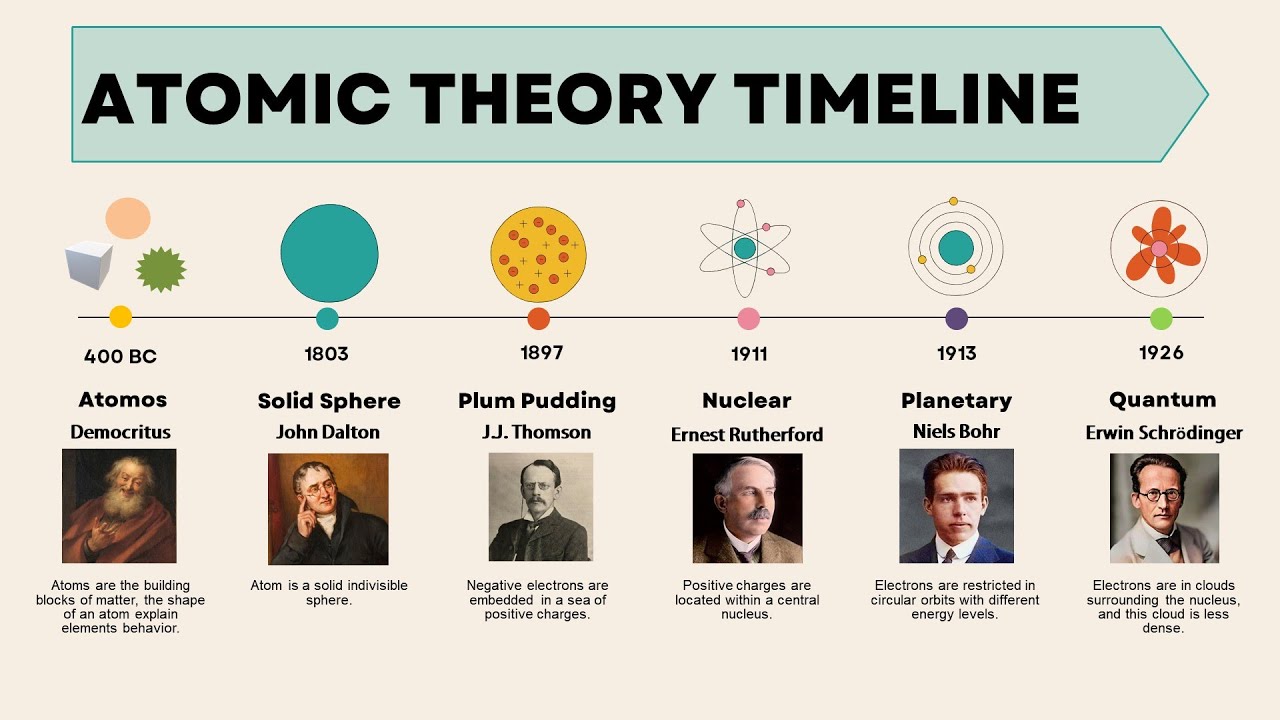
As scientific understanding grew, so did the atomic model:
- J.J. Thomson in 1897 discovered electrons, proving atoms are not indivisible but composed of smaller particles. He proposed the plum pudding model, where electrons (plums) are embedded in a positively charged sphere (pudding).
- Ernest Rutherford, through his gold foil experiment in 1911, introduced the nuclear model. Rutherford determined that atoms have a dense, positively charged nucleus at their center, with electrons orbiting around it. This was a radical shift from Thomson’s view.
Quantum Mechanics and Atomic Structure

The 20th century brought quantum mechanics, leading to more sophisticated views of atoms:
- Niels Bohr refined atomic models with his model in 1913, where electrons travel in circular orbits around the nucleus. However, his model didn’t account for electron interactions.
- Louis de Broglie and later Erwin Schrödinger proposed that electrons exhibit wave-particle duality, leading to the quantum mechanical model. This model uses probability distributions to describe electron positions, fundamentally changing our understanding of atomic behavior.
Modern Atomic Theory

Current atomic theory synthesizes these discoveries:
- Atoms are now seen as clouds of probability where electrons exist, governed by principles of quantum mechanics.
- Atoms have been manipulated, with scanning tunneling microscopes allowing scientists to move and observe individual atoms, verifying quantum theories.
- The Standard Model of Particle Physics explains the structure of subatomic particles, like quarks forming protons and neutrons, enhancing our atomic understanding.
The journey from antiquity's atomos to today's quantum view of atoms showcases the evolving nature of scientific inquiry. Each step in atomic theory development wasn't just about refining our understanding of matter but was also about challenging our perceptions of reality itself. This history not only illuminates the persistent quest to understand the nature of matter but also underscores the importance of questioning, experimenting, and refining theories.
What was the significance of John Dalton’s contribution to atomic theory?

+
Dalton’s atomic theory was pivotal because it provided the first systematic explanation of chemical combinations in terms of atomic weights and proportions, fundamentally changing chemistry and our understanding of how matter behaves.
How did Rutherford’s gold foil experiment change the atomic model?

+
Rutherford’s experiment revealed that atoms have a dense, positively charged nucleus surrounded by a cloud of electrons, drastically different from the previously held model of an evenly distributed charge, marking the shift from a pudding-like structure to one with a clear distinction between nucleus and electron cloud.
What role does quantum mechanics play in modern atomic theory?

+
Quantum mechanics introduced the concept that electrons behave both as particles and waves, leading to models where electron locations are described by probability distributions, fundamentally altering our view of atomic structure and behavior.
Why was the discovery of the neutron significant?

+
The discovery of the neutron by James Chadwick in 1932 completed the basic picture of the atomic nucleus, providing an explanation for nuclear stability, radioactive decay, and the basis for further nuclear physics research.