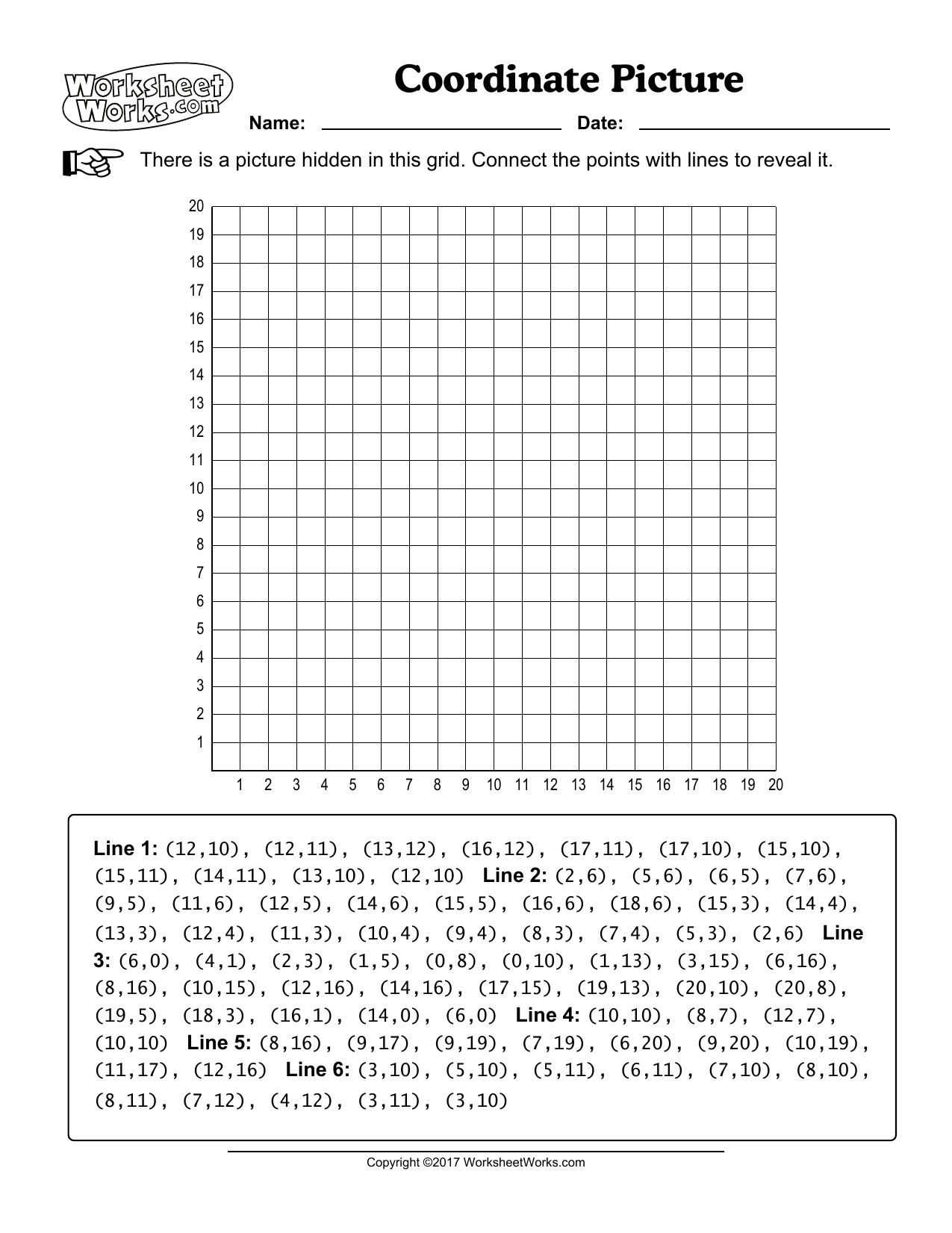
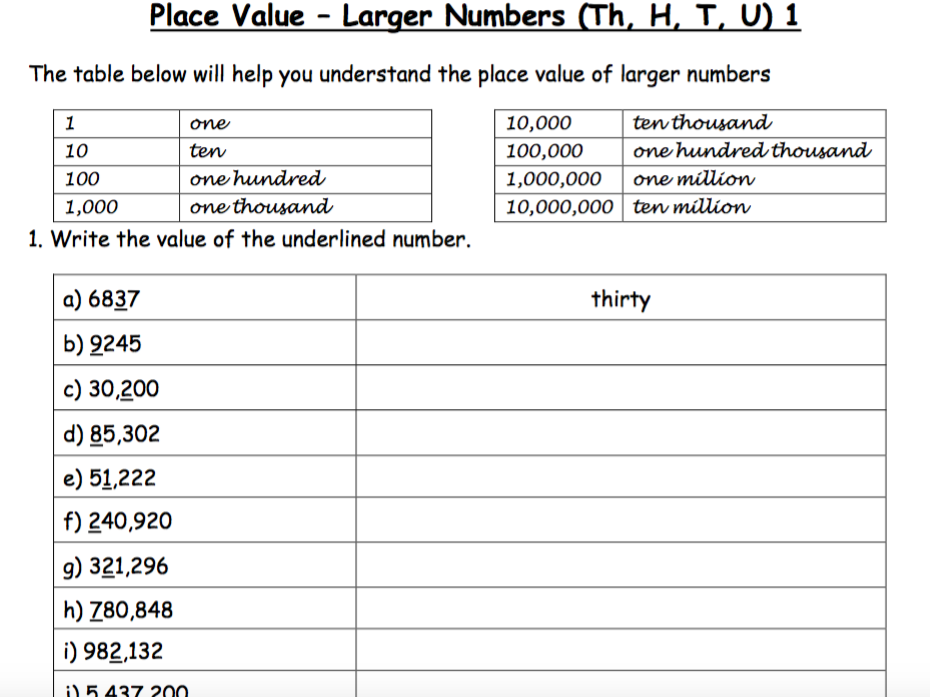
History of an Atom Worksheet: Fascinating Journey Revealed
In the realm of science, few topics have captivated the imagination quite like the concept of the atom. From the earliest musings of ancient philosophers to the cutting-edge experiments in modern quantum physics, the history of atomic theory is a compelling narrative of human curiosity and discovery. Let's embark on this fascinating journey, exploring how the atom went from a philosophical idea to a cornerstone of scientific understanding.
The Ancient Roots: From Philosophy to Pre-Science


Our journey begins with the ancient Greeks, over 2,500 years ago. Here are the key milestones:
- Leucippus and Democritus: These philosophers were among the first to suggest that matter is made up of indivisible particles they called 'atomos,' meaning uncuttable or indivisible.
- Epicurean Atomism: Later, Epicurus refined this idea, positing that atoms move randomly through the void, occasionally colliding to form the world we observe.
Despite these philosophical roots, there was no empirical evidence to support atomic theory until much later. Instead, the ideas of Plato and Aristotle, who suggested a more continuous, changeable nature of matter, dominated for centuries.
💡 Note: Early atomic theories were philosophical in nature, lacking empirical backing, which highlights the slow transition from metaphysical speculation to empirical science.
The Renaissance and Early Modern Ideas


The medieval and Renaissance periods saw a resurgence of interest in the ancient philosophies, setting the stage for modern atomic theory:
- Roger Bacon: An English philosopher who, while not explicitly theorizing about atoms, contributed to experimental science.
- Pierre Gassendi: Revived Epicurean atomism, integrating it with Christian theology, suggesting God created atoms.
- Robert Boyle: Through his work, "The Sceptical Chymist," Boyle challenged the prevailing Aristotelian theory of the four elements, advocating for a corpuscular theory of matter.
The Birth of Modern Chemistry: Dalton and Beyond


John Dalton's contributions in the late 18th and early 19th centuries marked a turning point:
- Dalton's Atomic Theory: Proposed that all matter is composed of atoms, which are indivisible and indestructible, and different elements have different weights and combine in whole numbers.
- Chemical Proportions: His law of multiple proportions became a pillar for understanding chemical reactions in terms of atomic theory.
💡 Note: Dalton's work was among the first to combine empirical observation with the concept of atoms, ushering in the age of modern chemistry.
The Atomic Revolution: Discoveries and Refinements

The 19th century was a period of rapid advancements:
- J.J. Thomson’s Electron: Through cathode ray experiments, Thomson discovered the electron in 1897, disproving the idea that atoms were indivisible.
- Ernest Rutherford’s Nucleus: His gold foil experiment in 1911 led to the idea of a dense atomic nucleus, with electrons orbiting around it.
- Niels Bohr’s Model: Integrated quantum theory with atomic structure, proposing discrete energy levels for electrons.
| Scientist | Contribution | Year |
|---|---|---|
| J.J. Thomson | Discovery of the electron | 1897 |
| Ernest Rutherford | Discovery of the nucleus | 1911 |
| Niels Bohr | Quantum model of the atom | 1913 |
The Quantum Leap: From Subatomic Particles to Quantum Mechanics


The 20th century's leaps in understanding quantum mechanics further reshaped our view of the atom:
- Werner Heisenberg and Erwin Schrödinger: Developed foundational theories for quantum mechanics, focusing on wave functions and probability distributions of electrons.
- Paul Dirac: Proposed the existence of antimatter, leading to the discovery of the positron.
- Subatomic Particles: The atom was now understood to consist not only of protons, neutrons, and electrons, but also quarks, leptons, and a host of other particles.
This journey through the history of the atom underscores the transformation from philosophical speculation to rigorous scientific inquiry. Each step forward in our understanding was built upon the groundwork laid by previous thinkers, pushing us ever closer to comprehending the true nature of the microscopic world.
Why did early Greek philosophers believe in atoms?

+
Ancient Greek philosophers like Leucippus and Democritus formulated the idea of atoms as a way to explain the nature of matter and change without invoking supernatural forces. They imagined that all material phenomena could be explained by the different arrangements of tiny, indivisible particles.
How did Dalton’s atomic theory differ from ancient theories?

+
John Dalton’s theory added a chemical framework to the atomic concept by asserting that atoms are not only indivisible but also combine in simple numerical ratios to form compounds. This idea was supported by empirical data, unlike the speculative nature of ancient theories.
What are the implications of quantum mechanics on our understanding of atoms?

+
Quantum mechanics introduced the probabilistic nature of subatomic particles, moving away from the deterministic view of the atom. It describes the behavior of electrons in terms of probability clouds rather than fixed orbits, fundamentally changing how we view the stability and interactions of matter.