Hess's Law Chem Worksheet 16.5 Answers Revealed

Delving into the world of chemistry, especially thermodynamics, can be both fascinating and complex. One principle that often comes into play is Hess's Law, which states that the total enthalpy change for a reaction is the same whether the reaction occurs in one or several steps. Today, we will unravel the answers to worksheet 16.5, providing you with not just the solutions but also a deeper understanding of the principles behind them.
Understanding Hess’s Law

Before we jump into the solutions, let’s briefly revisit what Hess’s Law entails:
- Enthalpy is a state function; its value depends only on the state of the system, not on the path taken to reach that state.
- The total enthalpy change for a chemical reaction can be determined by adding the enthalpies of intermediate reactions that sum to the overall reaction.
Problem 1: Direct Application of Hess’s Law
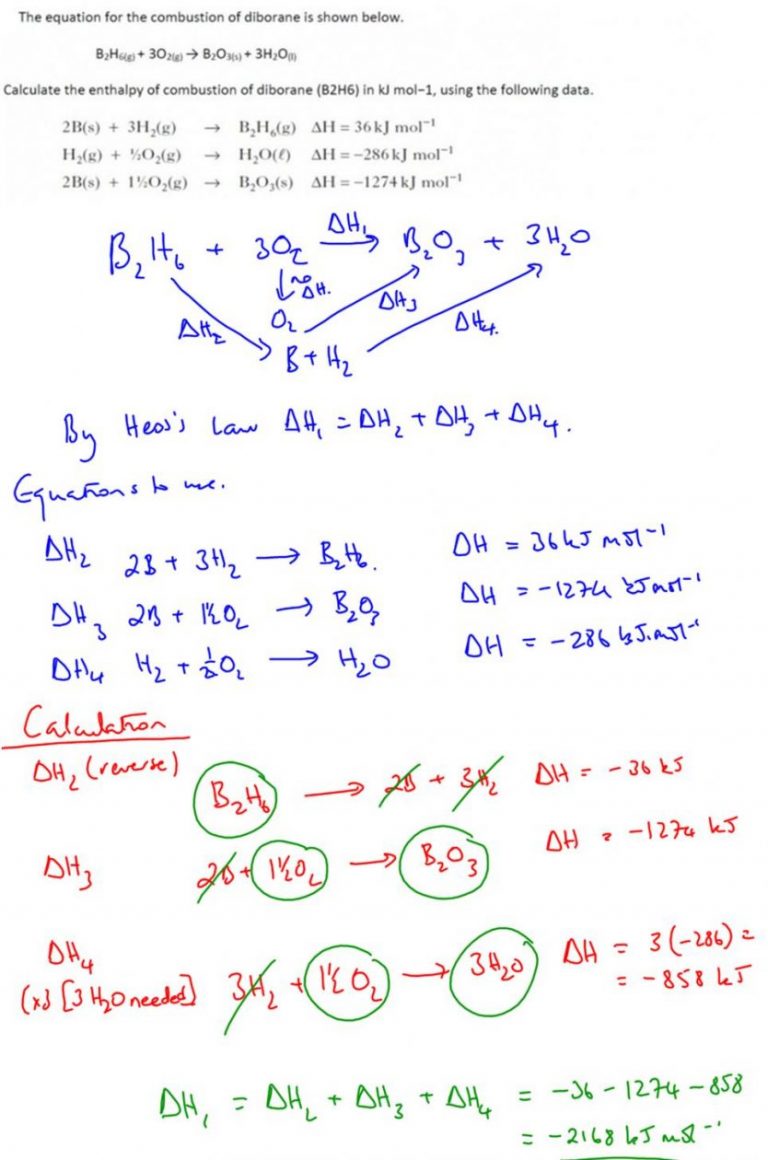
Question: Given the following reactions and their enthalpy changes:
- 2H2(g) + O2(g) → 2H2O(g) ΔH = -483.6 kJ
- CO(g) + 1/2O2(g) → CO2(g) ΔH = -283.0 kJ
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) ΔH = -890.4 kJ
Solution: We can approach this by:
- Writing down the given reactions and adjusting the equations to cancel out common substances.
| Reaction | Manipulations | Enthalpy (kJ) |
|---|---|---|
| 2H2(g) + O2(g) → 2H2O(g) | None | -483.6 |
| CO(g) + 1/2O2(g) → CO2(g) | Multiply by -1 | 283.0 |
| CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) | None | -890.4 |
| Result | CH4(g) + O2(g) → CO(g) + 2H2O(g) | -210.8 |

The enthalpy change for the reaction is -210.8 kJ.
🔍 Note: When manipulating equations, remember to multiply or divide the enthalpy change by the same factor to maintain the correct ratio.
Problem 2: Multiple Pathway Analysis

Question: Given:
- N2(g) + 2O2(g) → 2NO2(g) ΔH = 66.4 kJ
- NO(g) + 1/2O2(g) → NO2(g) ΔH = -57.1 kJ
Find the enthalpy change for 2NO(g) → N2(g) + O2(g).
Solution:
- First, reverse the second reaction to produce NO from NO2:
- NO2(g) → NO(g) + 1/2O2(g) ΔH = 57.1 kJ
- Multiply this equation by 2 to match the target equation:
- 2NO2(g) → 2NO(g) + O2(g) ΔH = 114.2 kJ
- Combine with the first reaction to cancel out NO2:
| Reaction | Manipulations | Enthalpy (kJ) |
|---|---|---|
| N2(g) + 2O2(g) → 2NO2(g) | None | 66.4 |
| 2NO2(g) → 2NO(g) + O2(g) | None | 114.2 |
| Result | 2NO(g) → N2(g) + O2(g) | 180.6 |
The enthalpy change for this reaction is 180.6 kJ.
Problem 3: Finding a Reaction Path

Question: Given the following reactions:
- C(graphite) + O2(g) → CO2(g) ΔH = -393.5 kJ
- CO(g) + 1/2O2(g) → CO2(g) ΔH = -283.0 kJ
Find the enthalpy change for the reaction C(graphite) + 1/2O2(g) → CO(g).
Solution:
- Reverse the second reaction and adjust for coefficient balancing:
- CO2(g) → CO(g) + 1/2O2(g) ΔH = 283.0 kJ
- Combine with the first reaction:
| Reaction | Manipulations | Enthalpy (kJ) |
|---|---|---|
| C(graphite) + O2(g) → CO2(g) | None | -393.5 |
| CO2(g) → CO(g) + 1/2O2(g) | None | 283.0 |
| Result | C(graphite) + 1/2O2(g) → CO(g) | -110.5 |
The enthalpy change for this reaction is -110.5 kJ.
🔹 Note: Always check if the equations can be balanced to match the target equation to avoid errors in calculation.
Wrapping Up

Through this exploration, we’ve seen how Hess’s Law serves as a powerful tool in thermodynamics, allowing us to calculate enthalpy changes for reactions that might be experimentally difficult or impossible to perform directly. By understanding the principle and practicing with worksheets like 16.5, you gain the ability to:
- Manipulate chemical equations to find an enthalpy pathway that suits the reaction in question.
- Use the enthalpy of formation data or given reactions to determine the heat of reaction for complex processes.
- Recognize the importance of balancing equations and considering stoichiometry when calculating enthalpy changes.
This knowledge not only aids in academic pursuits but also has practical applications in various fields like chemical engineering, energy studies, and environmental science where enthalpy changes dictate process efficiency, heat management, and energy conservation strategies.
Why is Hess’s Law Important?

+
Hess’s Law allows us to calculate the heat of reaction for reactions that are not easy to measure directly by summing the enthalpy changes of simpler, known reactions. This is vital in fields where knowing energy changes is key to efficiency and environmental impact assessments.
Can Hess’s Law be applied to any reaction?

+
Yes, because enthalpy is a state function, Hess’s Law applies to any reaction pathway, whether simple or complex, as long as the reactions can be algebraically manipulated to sum to the target reaction.
How does Hess’s Law help in predicting the spontaneity of a reaction?
+
While Hess’s Law directly deals with enthalpy changes, knowledge of these changes can be combined with entropy considerations to predict reaction spontaneity using Gibbs free energy calculations, where ΔG = ΔH - TΔS.
What are some common mistakes when applying Hess’s Law?

+
Common errors include failing to balance the chemical equations properly, not considering the stoichiometry of reactants and products, and incorrect sign manipulation when reversing reactions or changing their coefficients.