5 Essential Tips for Mastering Heating Curves

Understanding the behavior of substances as they change in temperature is fundamental in both physical chemistry and material science. Heating curves are graphical representations that illustrate these changes, providing insights into phase transitions, heat capacities, and thermal energy transfers. In this in-depth guide, we'll explore five crucial tips to help you master the intricacies of heating curves, ensuring your grasp on this topic is both thorough and applicable.
Tip 1: Grasp the Fundamentals of Phase Changes

Before delving into heating curves, one must have a clear understanding of the phase changes. Here's a quick reminder:
- Solid to Liquid (Melting): The energy is absorbed to break the forces holding the solid in place, but temperature remains constant.
- Liquid to Gas (Boiling or Evaporation): Heat energy is used to transition molecules into a gas state, again with no increase in temperature.
- Sublimation: Direct transition from solid to gas, skipping the liquid phase entirely.
- Deposition: Direct transition from gas to solid.
The understanding of these transitions is key to interpreting heating curves, where horizontal segments represent the addition of heat without a change in temperature due to phase changes.
🔎 Note: Remember that energy input does not necessarily increase temperature during phase changes.
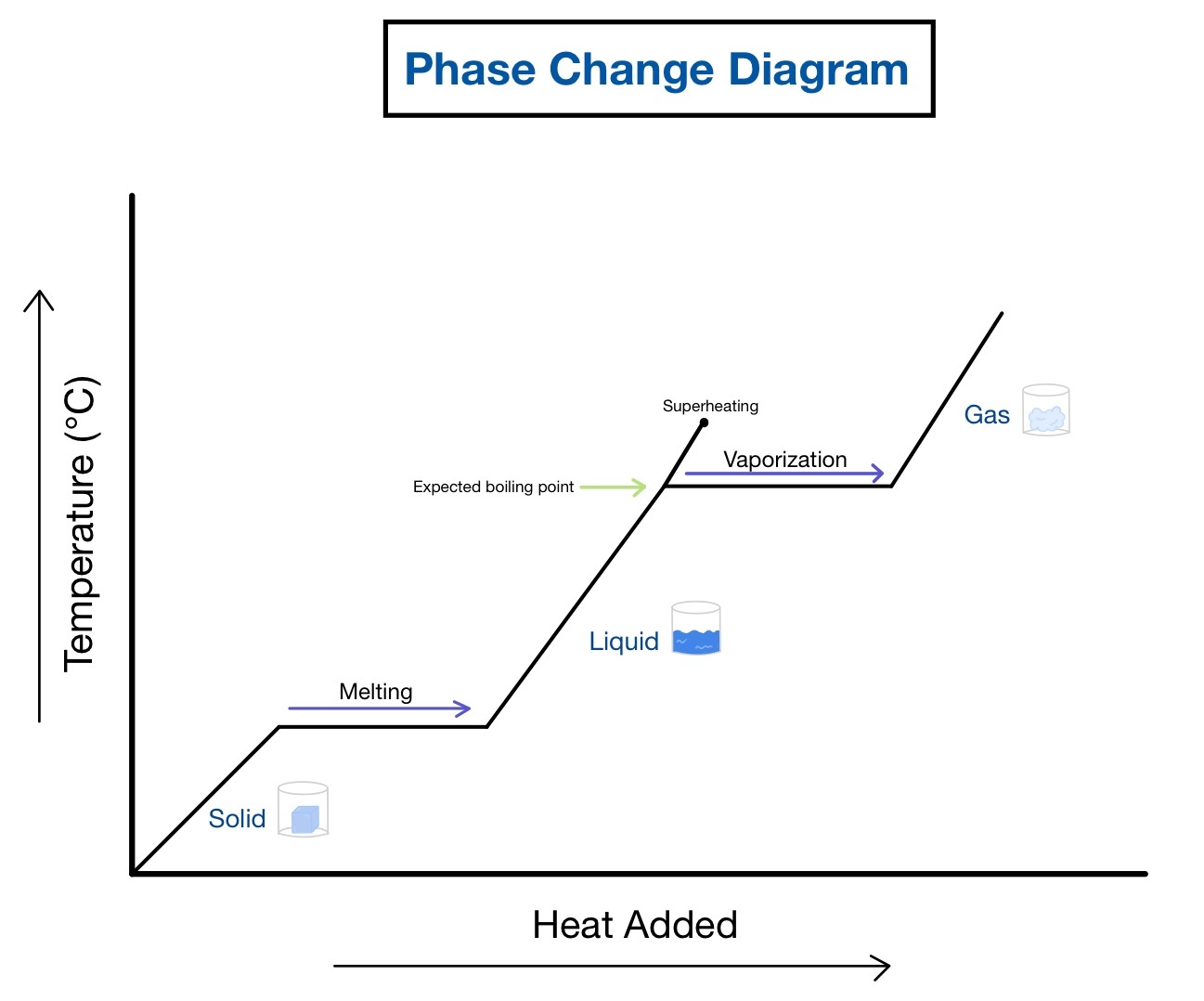
Tip 2: Identify Different Regions in the Curve


Heating curves often feature distinct regions:
- Initial heating of the solid: A rising diagonal line where the substance’s temperature increases.
- Melting: A flat line where the solid is becoming liquid at the melting point.
- Heating of the liquid: Another diagonal rise as the liquid’s temperature increases.
- Boiling: A flat line where the liquid changes to a gas at the boiling point.
- Heating of the gas: Finally, another diagonal rise until the experiment ends.
📚 Note: Not all heating curves will show all regions. The phase of the substance at the beginning of the experiment will determine the visible phases.
Tip 3: Determine Heat Capacity from the Slopes

The slope of each region on the heating curve can reveal the substance’s heat capacity:
- Solids: The slope is generally smaller, indicating a lower heat capacity because solids have tightly packed molecules.
- Liquids: The slope is typically steeper, reflecting a higher heat capacity as molecular movements become more chaotic.
- Gases: The slope is the steepest due to the much higher heat capacity and the freedom of movement of gas molecules.
💡 Note: The heat capacity can be calculated from the slope (ΔT / Δq) where ΔT is temperature change, and Δq is heat added.
Tip 4: Calculate Energy of Phase Change

Heat Energy Calculations for Phase Changes
| Phase Change | Symbol | Heat Involved (J/g) |
|---|---|---|
| Melting (Ice to Water) | ΔHm | 334 |
| Boiling (Water to Steam) | ΔHv | 2260 |

To find the energy required for phase changes:
- Use the formula: Q = mΔH (where Q is energy, m is mass, and ΔH is the enthalpy of the phase change).
- During the phase change, no temperature change occurs, so energy only goes into breaking molecular bonds.
✏️ Note: Enthalpy values can be found in tables for specific substances or experimentally determined.
Tip 5: Analyze the Heating Curve for Substance Identification
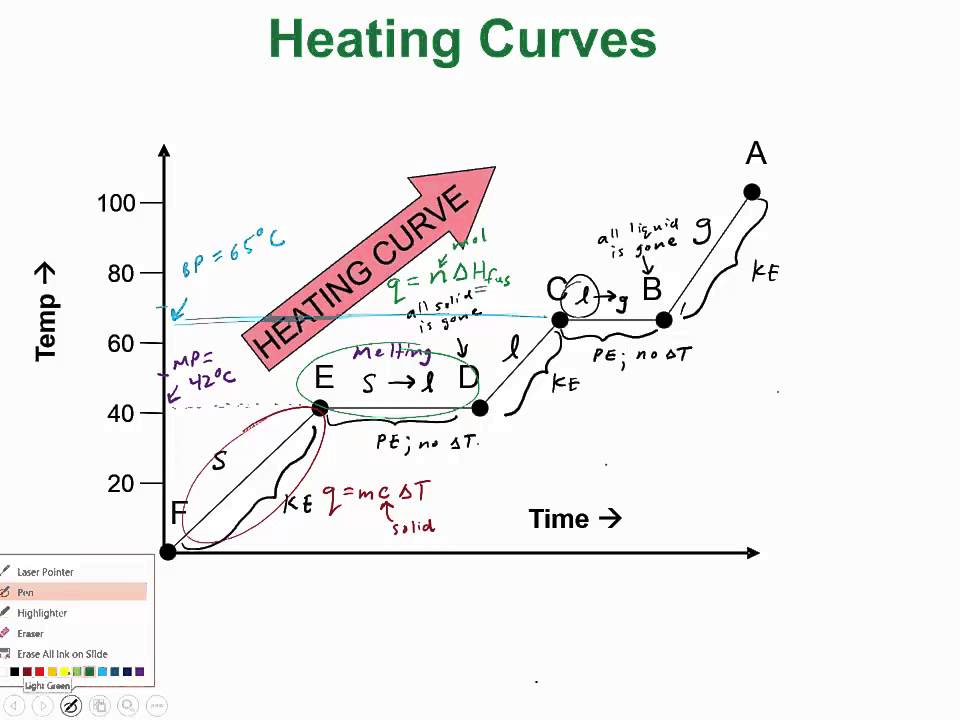
By analyzing heating curves, you can:
- Identify the substance: Using known melting and boiling points.
- Assess purity: Impurities lower melting points and increase the range over which the phase change occurs.
- Determine the phases present at any point: This helps in understanding the state of the substance at different temperatures.
🔬 Note: Real-world curves may have slight deviations due to impurities, pressure, and other factors.
In conclusion, mastering heating curves involves understanding the physics behind them, accurately interpreting the different regions, calculating heat capacities and energies, and applying this knowledge to identify substances and assess their purity. Whether you are a student, researcher, or professional, these tips will provide you with the tools to navigate this fundamental aspect of thermodynamics with confidence and precision.
What causes the horizontal lines on a heating curve?

+
The horizontal lines on a heating curve represent phase changes, where the added energy is used to overcome the forces keeping the molecules together, rather than increasing the temperature of the substance.
Can you determine a substance’s identity from its heating curve?

+
Yes, by knowing the melting and boiling points, which are indicated by the flat lines on the heating curve, you can compare these with known values to identify the substance.
Why do heating curves sometimes not show all regions?
+
The regions on a heating curve depend on the starting state of the substance. For example, if you start with ice, you might see melting and boiling but not a freezing point because the experiment ended before that temperature was reached.