5 Key Answers to Heating Curve Worksheet Explained

Understanding a heating curve is essential for anyone studying thermodynamics, chemistry, or any scientific field involving changes in state. Here, we delve into five key answers that demystify the heating curve, providing insights that not only answer common questions but also enhance understanding for practical applications.
1. What is a Heating Curve?

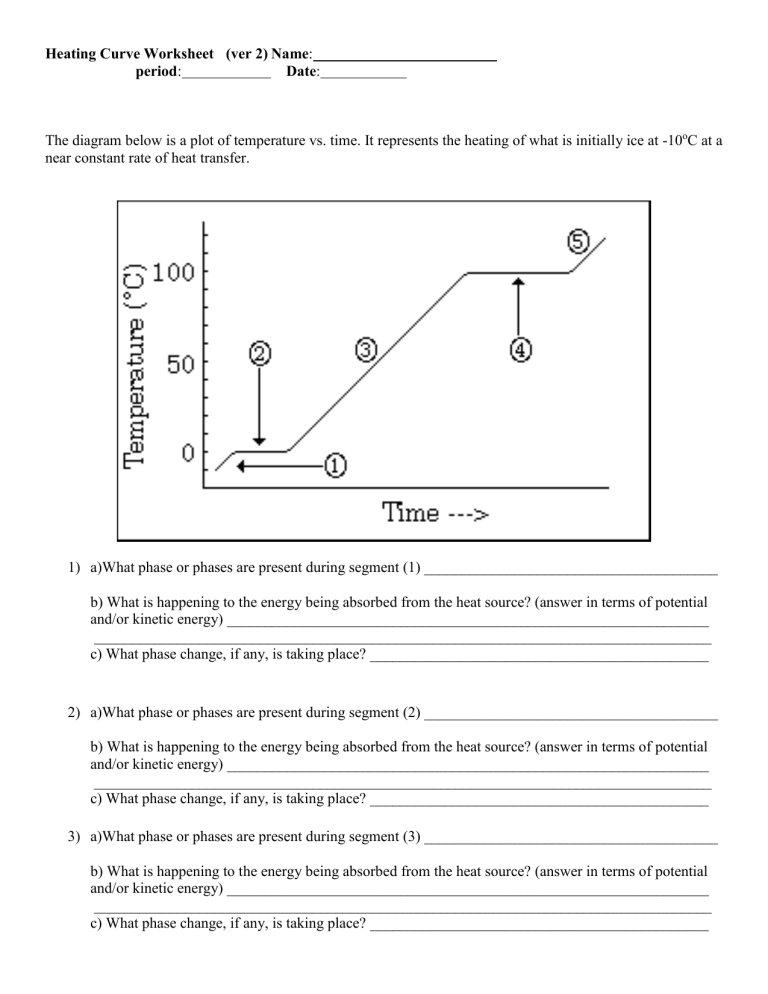
A heating curve represents the changes in temperature over time as heat is added to a substance. Here’s what happens:
- Solid to Liquid: The substance begins in a solid state, and as heat is added, the temperature rises until it reaches the melting point where it plateaus. During this phase, the energy supplied goes into breaking molecular bonds, not increasing temperature.
- Liquid: After melting, the temperature of the liquid increases until it reaches the boiling point.
- Liquid to Gas: At the boiling point, the temperature plateaus again as the heat supplied goes into vaporizing the liquid, overcoming intermolecular forces.
- Gas: Once fully vaporized, further addition of heat increases the temperature of the gas.
📝 Note: The shape of a heating curve can vary depending on the substance because the melting and boiling points differ. Each phase change requires specific amounts of energy known as the heat of fusion (solid to liquid) and heat of vaporization (liquid to gas).
2. How Does Heat Affect the Phases of Matter?
Heat affects phases of matter by providing the necessary energy for phase transitions:
- Melting (Fusion): Heat breaks the forces holding atoms or molecules in a crystalline lattice, allowing them to move more freely as a liquid.
- Boiling (Vaporization): Additional heat disrupts the attractions between molecules, allowing them to escape into the gas phase.
- Heat Absorption: During phase changes, energy is absorbed or released, but the temperature remains constant due to the energy going into or coming from the phase change itself.
3. Why Does the Temperature Remain Constant During Phase Changes?

When a substance is undergoing a phase change, the energy supplied is used to:
- Disrupt intermolecular forces or create new bonds.
- Work against the latent heat of fusion or vaporization.
The temperature doesn’t increase because the energy is used to change the phase, not to increase kinetic energy. Once the phase change is complete, the temperature can resume rising.
4. Interpreting Slopes in Heating Curves

| State | Slope | Interpretation |
|---|---|---|
| Solid | Rising | The temperature increases as heat is added. |
| Melting | Flat | The temperature remains constant as heat melts the solid. |
| Liquid | Rising | As more heat is added, the liquid's temperature rises. |
| Boiling | Flat | The temperature is steady while the liquid converts to gas. |
| Gas | Rising | The temperature increases as heat is added to the gas. |

🧪 Note: The slope during the solid, liquid, and gas phases represents the rate of temperature change, which is a reflection of the specific heat capacity of the substance in each phase.
5. Practical Applications of Heating Curves

Heating curves are not just theoretical tools but have numerous practical applications:
- Cooking: Understanding how heat affects phase changes can help you master cooking techniques like melting chocolate or steaming food without overcooking.
- Industrial Processes: Industries use heating curves to control phase changes in materials, such as melting metals or vaporizing liquids for manufacturing.
- Weather Forecasting: Meteorologists use principles similar to heating curves to predict phenomena like freezing rain or fog.
By wrapping up our exploration of the heating curve, we've gained a clearer picture of how heat influences the physical state of matter. These principles not only help in academic settings but are also invaluable in everyday applications. Whether you're cooking, analyzing industrial processes, or interpreting weather forecasts, the heating curve provides essential insights into how substances behave under thermal stress.
What is the difference between heat capacity and specific heat?

+
Heat capacity is the amount of heat required to raise the temperature of an entire substance by one degree Celsius or Kelvin, whereas specific heat is the amount of heat required to raise the temperature of one unit mass of a substance by one degree. Specific heat is typically used when dealing with specific substances.
Why is the heating curve useful in chemical processes?

+
The heating curve provides a visual representation of phase changes and temperature stability, allowing chemists to predict and control processes where specific temperature conditions are critical. It’s especially useful in reactions that involve heat transfer or change of state.
How do impurities affect a heating curve?

+
Impurities generally lower the melting point and widen the range over which the substance melts, causing the plateaus on the heating curve to start at a lower temperature and span a broader range, making the curve less sharp.