5 Essential Answers for Water Heating Curve Worksheet

Introduction to Water Heating Curve Worksheet

When we dive into the realm of physical science, understanding how substances change phase and the energy dynamics involved is key. A water heating curve is an excellent educational tool for students, scientists, and engineers alike, providing insights into the thermodynamics of water. This blog post will guide you through the five essential answers for working on a Water Heating Curve Worksheet, helping you grasp how to calculate heat changes, interpret phase transitions, and understand the significance of latent heat.
The Concept of a Heating Curve
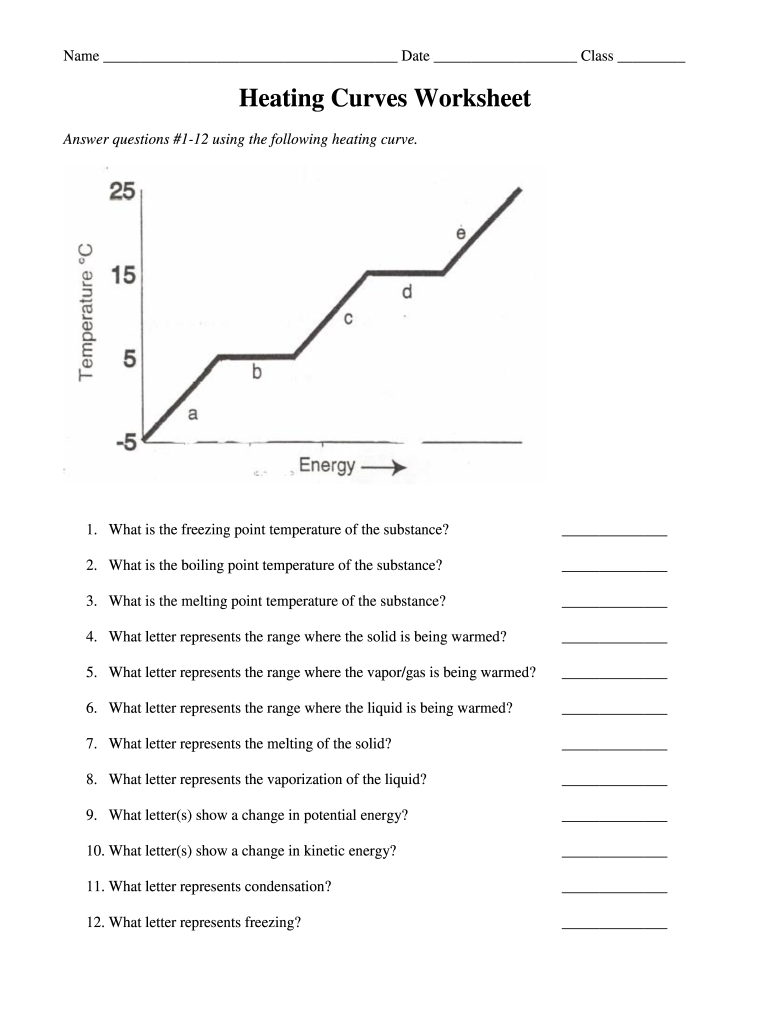
A heating curve graphically represents the change in temperature of a substance when heat is applied over time. Here are the fundamental concepts associated with water:
- Heat Input: Energy added to water can either increase its temperature or change its state without increasing its temperature.
- Temperature Plateaus: During phase transitions (melting or boiling), water’s temperature stays constant due to the energy used for breaking molecular bonds rather than increasing molecular movement.
- Energy Calculations: The heat energy required to raise the temperature or change the phase can be calculated using specific heat capacity and latent heat values.

Answer 1: Calculating Heat Needed to Raise Temperature

To find the heat required to raise the temperature of water from one point to another, you use the formula:
Q = mcΔTWhere:
- Q is the heat energy in joules (J)
- m is the mass of water in grams (g)
- c is the specific heat capacity of water (4.184 J/g°C)
- ΔT is the change in temperature (°C)
🔹 Note: Always ensure units are consistent when performing calculations.
Answer 2: Determining Energy for Phase Transitions

When water changes state (ice to liquid water or liquid water to vapor), heat energy is used to break or form hydrogen bonds. The equation for phase transitions is:
Q = mLWhere:
- Q is the heat energy in joules (J)
- m is the mass of water in grams (g)
- L is the latent heat of fusion (for melting ice, 334 J/g) or latent heat of vaporization (for boiling, 2256 J/g)
🔹 Note: Latent heat is not used to increase temperature but to change the phase of the substance.
Answer 3: Interpreting the Graph

Understanding the graph involves observing the following key features:
- Solid to Liquid: A horizontal plateau at 0°C indicating the ice melting (latent heat of fusion being absorbed).
- Liquid Heating: An upward slope where heat is raising the temperature of liquid water.
- Liquid to Vapor: Another horizontal plateau at 100°C where water is boiling (latent heat of vaporization being absorbed).
- Vapor Heating: A less steep rise in temperature for steam heating.
Answer 4: Applying the Concepts to Real-World Scenarios

Understanding heating curves can be applied in:
- Heat pumps and heating systems.
- Cooking, where managing temperatures and phase changes is crucial.
- Climate control and energy efficiency studies.
- Forensic analysis to determine time of death based on body cooling.
Answer 5: Misconceptions to Avoid

Here are common misconceptions to keep in mind:
- Heat is not just a temperature increase; it can also facilitate phase change without altering temperature.
- The specific heat capacity changes with temperature, particularly during phase transitions.
- Heat energy is not “used up” but is transferred or transformed.
Summing Up Key Takeaways

When working with a water heating curve worksheet, remember to:
- Use the correct formulas for calculating heat energy in various processes.
- Account for the two types of energy changes: sensible heat for temperature change and latent heat for phase change.
- Interpret the graph to understand how energy affects temperature and phase transitions.
- Apply these concepts to practical, real-world applications.
- Be aware of common misconceptions to enhance your understanding of the heating curve phenomenon.
What is the difference between sensible and latent heat?

+
Sensible heat changes the temperature of a substance, while latent heat is absorbed or released during a phase change without changing the temperature.
Why does the temperature remain constant during phase transitions?

+
During phase changes, all the energy is used to overcome or form intermolecular forces, not to increase the kinetic energy of molecules, so the temperature stays constant.
Can water be superheated or supercooled?

+
Yes, water can exist in superheated (above boiling point without boiling) or supercooled (below freezing point without freezing) states under specific conditions.