5 Essential Tips for Understanding Heating Cooling Curves
Understanding heating and cooling curves is essential in thermodynamics and materials science. These curves illustrate the phase changes and temperature variations of substances under different conditions. Whether you're a student, a scientist, or someone interested in the physical properties of matter, mastering these concepts can significantly enhance your understanding of how materials behave. Here are five essential tips to help you decode these important graphs effectively.
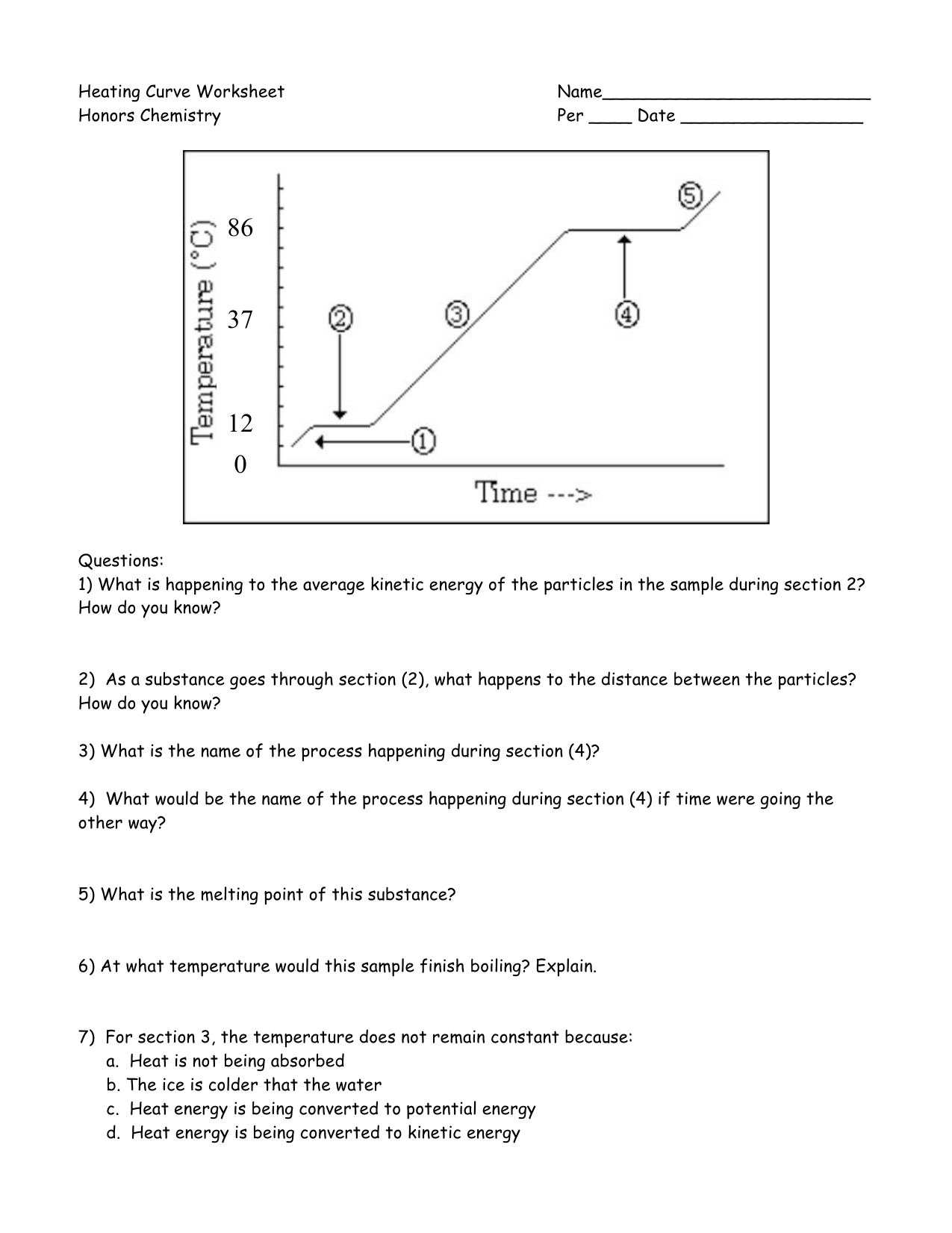
1. Recognize Key Points on the Curve

Heating and cooling curves are not just lines on a graph; they contain several critical points:
- Melting/Freezing Point: This is where the substance changes between solid and liquid states. At this point, the temperature remains constant despite adding or removing heat.
- Boiling/Condensation Point: Here, the transition is from liquid to gas or vice versa, with a similar constant temperature observation.
- Heat Capacity: Before reaching these transition points, the temperature increases or decreases linearly. This segment represents the heat capacity of the substance.
- Phase Equilibrium: During phase transitions, the substance reaches a state of equilibrium, where the rate of change from one phase to another is equal.
🔍 Note: Identifying these points accurately will give you insights into the substance's behavior under different thermal conditions.
2. Understand Enthalpy Changes

The phase changes are accompanied by changes in enthalpy (heat content) of the system:
- During melting or freezing, the enthalpy change is called latent heat of fusion.
- In the case of boiling or condensation, it's known as the latent heat of vaporization.
- These heats are absorbed or released without a change in temperature, which is why the curve plateaus.
Here’s a simple representation of how these heats are measured:
| Phase Transition | Enthalpy Term | Symbol | Units (J/g or kJ/kg) |
|---|---|---|---|
| Melting/Freezing | Latent Heat of Fusion | ΔHfus | Varies by substance |
| Boiling/Condensation | Latent Heat of Vaporization | ΔHvap | Varies by substance |

3. Apply Energy Conservation

Remember that the First Law of Thermodynamics holds true for heating and cooling curves:
- The energy added to or removed from a system is equal to the change in internal energy, which can manifest as temperature change or phase change.
- The formula q = mcΔT is useful here, where q is heat energy, m is mass, c is specific heat capacity, and ΔT is the change in temperature. During phase changes, ΔT = 0, so:
\[ q = mL_f \quad \text{(melting/freezing)} \] \[ q = mL_v \quad \text{(boiling/condensation)} \]
where Lf and Lv are the latent heats.
4. Consider the Slope of the Curve

The slope of the curve outside the phase transitions provides information on:
- Heat Capacity: A steeper slope indicates lower heat capacity because less heat is needed to change the temperature.
- Thermal Conductivity: This can influence how quickly temperature changes occur in the substance.
Analyzing the slope can reveal important material properties:
- Materials with a high specific heat capacity (e.g., water) have a flatter heating/cooling curve slope.
- Materials with good thermal conductivity will show a quicker temperature response, leading to a more pronounced slope.
🛠️ Note: The slope can also be affected by the rate at which heat is supplied or removed, known as the heating or cooling rate.
5. Interpret the Curve in Real-World Contexts

Heating and cooling curves have practical applications:
- Cooking: The melting point and boiling point help determine cooking temperatures.
- Material Processing: Knowing phase transitions is critical for manufacturing processes like casting or forging.
- Refrigeration: Understanding heat absorption during phase changes is key to designing effective refrigeration systems.
- Weather Analysis: The freezing and melting points of water are essential in predicting weather patterns.
By mastering these curves, you can better comprehend and manipulate various physical processes:
- In industrial applications, accurate phase change data allows for precise control of processes.
- In educational settings, this knowledge aids in teaching fundamental thermodynamics and material behavior.
🌍 Note: Real-world applications often require consideration of impurities, pressure changes, or other factors that can shift or modify the heating and cooling curves.
By following these essential tips, you'll develop a deeper understanding of heating and cooling curves. Not only will this aid in academic pursuits, but it will also empower you to make informed decisions in real-world scenarios where thermodynamics and materials science play a role. Whether optimizing heating systems or analyzing weather patterns, these principles will prove invaluable.
Why is the temperature constant during phase transitions?

+
The temperature stays constant because the added or removed heat energy is used to break or form bonds between the particles, not to change the temperature. This is due to the energy associated with latent heat.
What does a flat part in the heating curve indicate?

+
A flat segment on the heating curve signifies a phase transition where the temperature does not change, indicating that the energy input is being used for the latent heat of fusion or vaporization.
How can I use heating and cooling curves for material selection in manufacturing?

+
Heating and cooling curves provide insight into the thermal behavior of materials. By understanding the phase change points and heat capacities, you can choose materials for processes like casting, where controlling the melting and solidification temperatures is crucial.



