5 Key Insights: Heating Cooling Curve Worksheet Answers
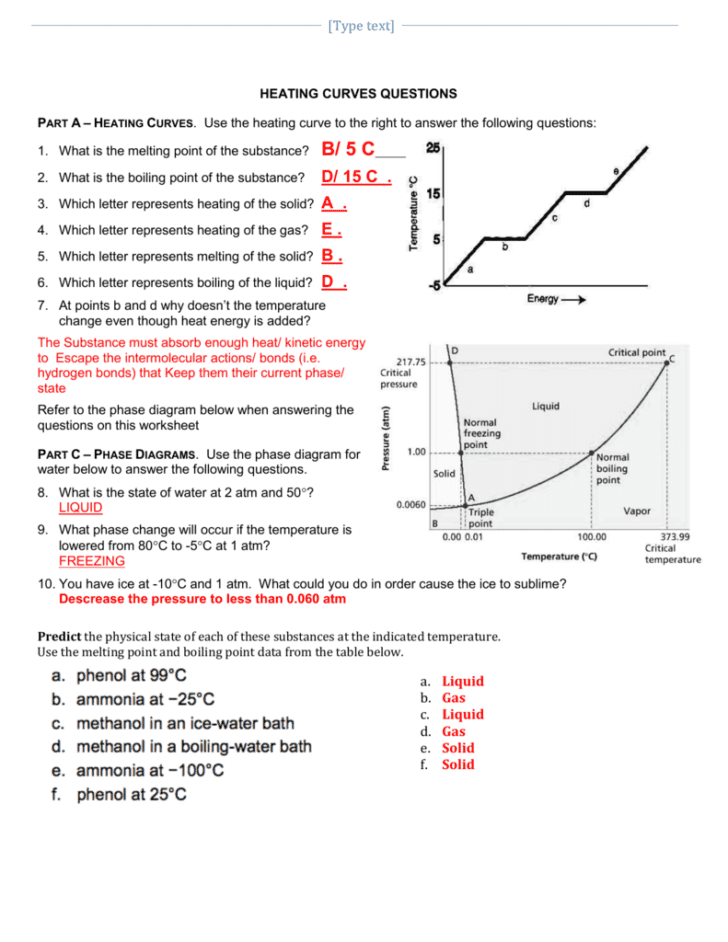
The process of understanding phase changes and the energy transformations involved is crucial for students studying physical sciences, chemistry, and engineering. A heating and cooling curve worksheet serves as a pedagogical tool to illustrate these transformations. Here, we delve into the nuances of such curves, providing key insights into interpreting and analyzing these changes in states of matter. Let's explore five critical areas:
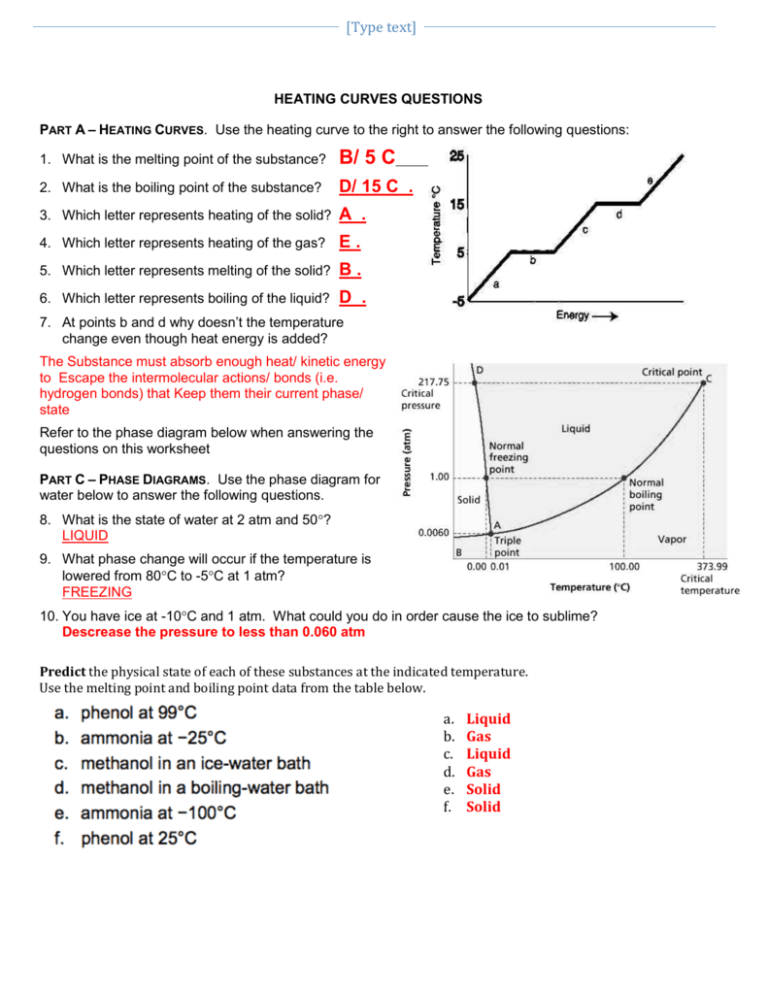
1. Understanding Phase Transitions

At the core of studying heating and cooling curves lies the grasp of phase transitions. When a substance gains or loses energy, it goes through various stages:
- Solid Phase: The material remains solid until it reaches its melting point.
- Melting Point: Energy added does not increase the temperature but causes the substance to melt into a liquid state.
- Liquid Phase: Here, the temperature increases as heat is added.
- Boiling Point: Additional heat causes the liquid to vaporize into a gas, but the temperature remains steady.
- Gas Phase: Finally, once the substance has completely vaporized, its temperature continues to rise with added heat.
These transitions are depicted as plateaus or flat lines on the heating or cooling curve, which signify constant temperature while the phase change occurs.
2. Analyzing Heat of Fusion and Heat of Vaporization
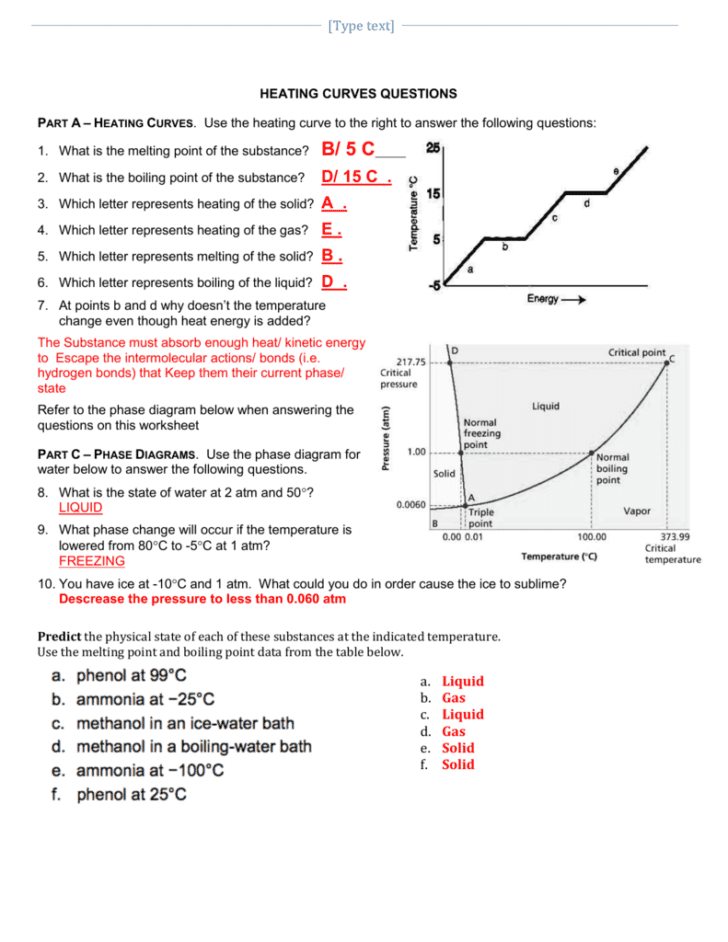
The heat of fusion and heat of vaporization are quantities of energy required to effect these phase changes without altering the temperature. On a worksheet, you will often be tasked with:
- Identifying where these changes occur on the curve.
- Calculating how much energy is involved in melting or boiling a given mass of substance.
The heat of fusion, for example, refers to the latent heat absorbed or released during melting or freezing. Similarly, the heat of vaporization is the energy involved in boiling or condensing the material.
3. Determining Temperature Changes
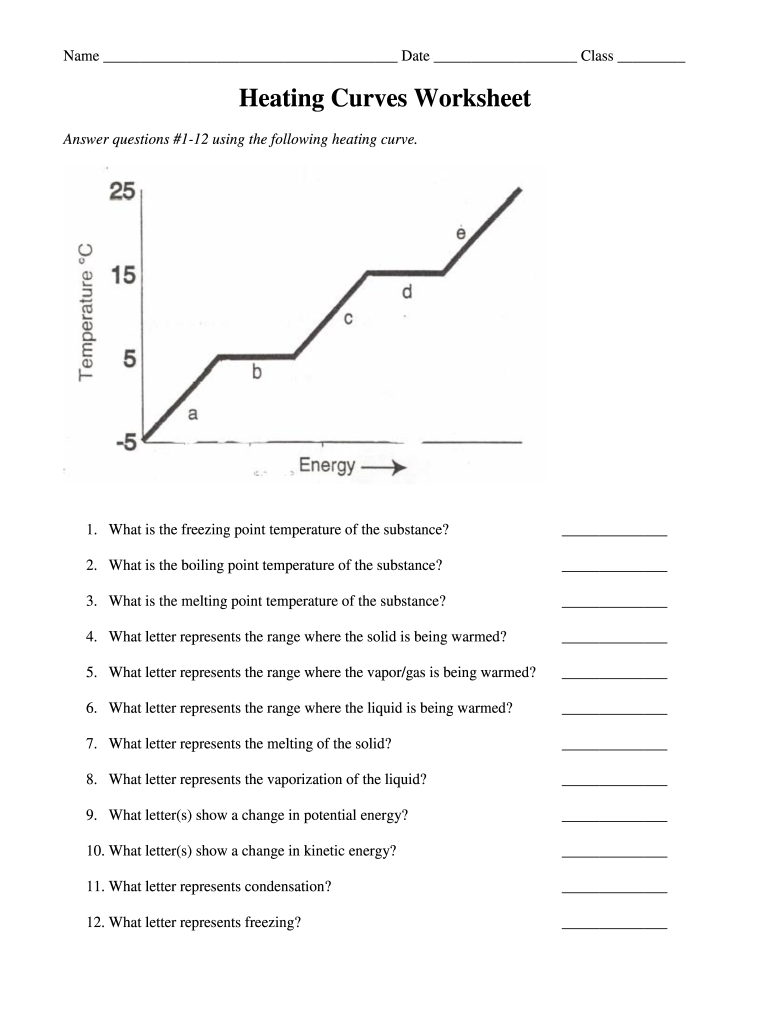
Once you’ve identified the phase transitions, understanding how temperature changes occur in each phase is imperative. Here’s what you should look for:
- On the sloping parts of the curve, the temperature changes, indicating energy input or loss directly affects the substance’s temperature.
- These changes can be calculated using formulas like q = mCΔT, where q is heat energy, m is mass, C is specific heat capacity, and ΔT is change in temperature.
4. Interpreting Graphs for Phase Diagrams
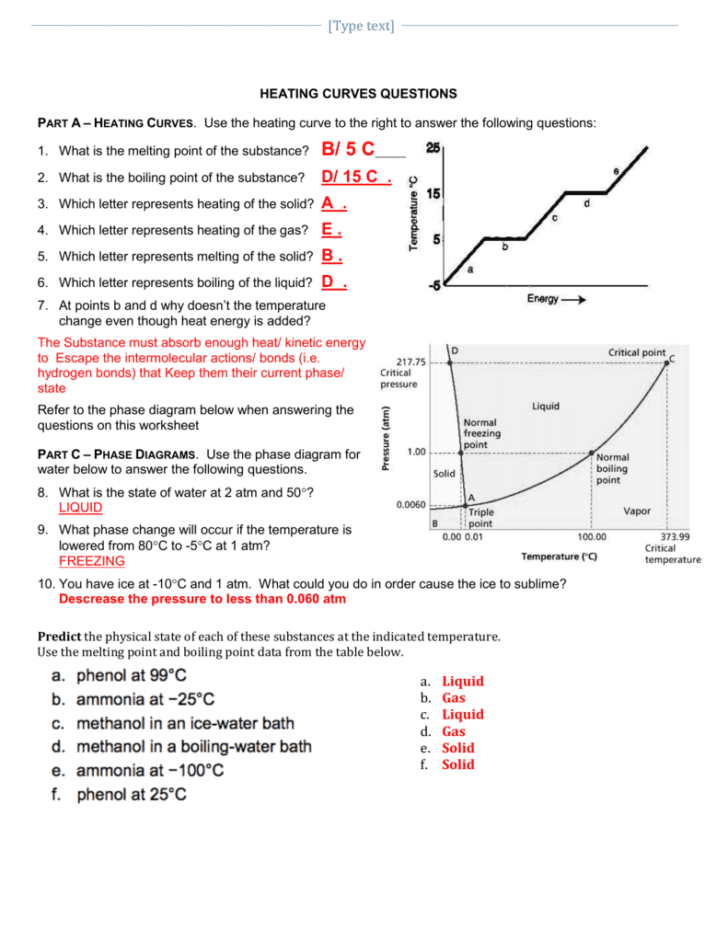
Often, heating and cooling curve worksheets will ask students to interpret graphs that closely relate to phase diagrams. Key points to consider are:
- The critical points where different phases coexist.
- The triple point where all three phases are in equilibrium.
- How pressure affects these transitions.
Such exercises help in visualizing the phase behavior under different conditions, a concept crucial for industrial processes like distillation or freeze-drying.
5. Applying Practical Insights

Heating and cooling curves are not just theoretical constructs. They have practical applications in:
- Chemical Engineering: For example, knowing when and how substances transition can help optimize the design of chemical reactors.
- Meteorology: Phase changes are vital in the formation of precipitation and weather patterns.
- Food Science: Understanding freezing and melting points can enhance food preservation techniques.
By applying these concepts, students can not only comprehend the physics but also recognize their real-world implications.
Finally, when interpreting these curves, consider the following:
🧠 Note: Always remember the specific heat capacity of different materials affects how rapidly temperature changes with heat addition or removal.
In summary, heating and cooling curves are a window into the dynamic equilibrium between energy and matter. They help in identifying phase transitions, understanding latent heats, calculating temperature changes, interpreting phase diagrams, and applying this knowledge in real-world scenarios. With this framework in mind, students can confidently approach related worksheets and deepen their understanding of matter's behavior in response to thermal energy.
What is the difference between heat of fusion and heat of vaporization?

+
Heat of fusion is the energy needed to change a solid to a liquid at its melting point, while heat of vaporization is the energy required to convert a liquid to a gas at its boiling point.
Why does the temperature remain constant during a phase transition?

+
During a phase transition, the energy supplied is used to weaken or break intermolecular forces, rather than to increase the kinetic energy (and thus the temperature) of the substance.
How can I calculate the amount of heat needed for a phase change?
+
Use the formula q = mΔH, where q is heat, m is the mass of the substance, and ΔH is either the heat of fusion or vaporization.
Can heating and cooling curves help predict weather patterns?

+
Yes, understanding phase transitions is key to predicting how moisture in the atmosphere will behave, affecting cloud formation, precipitation, and other weather phenomena.