5 Tips for Understanding Heating and Cooling Curves

Understanding heating and cooling curves is essential for students, researchers, and anyone working in fields where the study of materials or thermodynamics is involved. These curves provide visual insights into the behavior of substances as they are subjected to temperature changes, offering valuable information on phase changes, heat capacity, and energy transfer. Here are 5 tips to help you better understand and analyze heating and cooling curves:
1. Identify Phases and Transitions


When you first look at a heating or cooling curve, your primary task is to identify the different phases of the substance and the transitions between these phases. Here are some key points:
- Look for plateaus, which indicate where a substance is undergoing a phase transition, such as melting or boiling.
- Understand that during these transitions, the temperature remains constant as the energy provided is used to change the substance’s state rather than increase its temperature.
- Identify the points at which transitions occur, like the melting point or freezing point. These are critical because they indicate the temperature at which the phase change happens.
🔥 Note: Changes in state absorb or release energy without changing the substance’s temperature.
2. Relate Heat Changes to Temperature

While analyzing these curves, it’s important to relate the heat energy supplied or removed to the changes in temperature:
- When a substance is not undergoing a phase change, the increase or decrease in temperature is directly proportional to the heat supplied or removed. This relationship is captured by the equation Q = mcΔT, where Q is heat, m is mass, c is specific heat capacity, and ΔT is the temperature change.
- During a phase change, the heat energy supplied is used to alter the internal structure of the substance, thus the temperature doesn’t change.
The shape of the heating or cooling curve reflects how the substance absorbs or releases heat, providing clues about its specific heat capacity.
3. Use the Slope for Heat Capacity Information
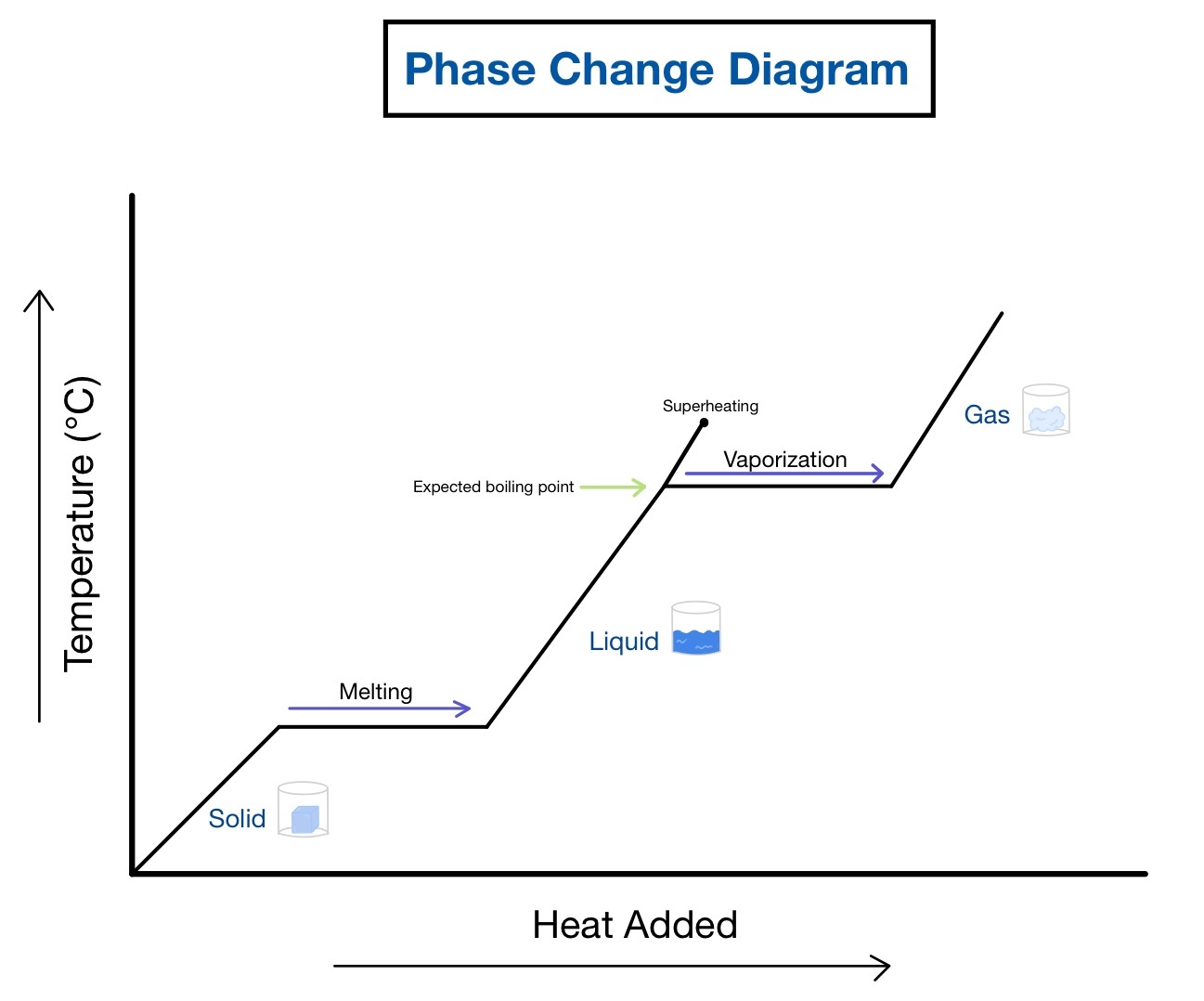
The slope of a heating or cooling curve, when the substance is in a single phase, can tell you about its specific heat capacity:
- Steeper slopes indicate a lower heat capacity because less temperature change occurs with the input of heat.
- Flat sections during phase changes mean that all energy input goes into changing the phase, not increasing the temperature.
This information helps you understand how different materials respond to heat and can be critical in designing systems or experiments involving temperature control.
4. Consider Heat of Fusion and Vaporization

The amount of heat absorbed or released during phase changes is governed by the substance’s heat of fusion (melting) or vaporization (boiling):
- Heat of fusion, represented by Q = mLfus, where Lfus is the latent heat of fusion.
- Heat of vaporization, represented by Q = mLvap, where Lvap is the latent heat of vaporization.
These values are material-specific and require careful consideration, especially in processes like distillation or melting point determinations.
| Material | Heat of Fusion (kJ/kg) | Heat of Vaporization (kJ/kg) |
|---|---|---|
| Water | 334 | 2260 |
| Alcohol | 109 | 855 |

5. Connect Curves to Practical Applications

Understanding heating and cooling curves has practical implications in:
- Designing efficient heating and cooling systems for buildings or industrial processes.
- Predicting the behavior of materials in different temperature environments.
- Analyzing energy transfers in chemical reactions or material processing, like in metallurgy or ceramics production.
By visualizing and analyzing these curves, you can make informed decisions that affect energy efficiency, material selection, and process optimization.
In summary, understanding heating and cooling curves involves identifying phase changes, analyzing temperature variations in relation to heat, using slopes for heat capacity information, considering latent heats, and applying this knowledge practically. Whether for academic studies or industrial applications, these principles can enhance your grasp of thermodynamics, energy transfer, and material properties. Embracing these tips will not only aid in academic success but also in practical problem-solving and innovation in related fields.
What is the significance of flat regions on heating and cooling curves?

+
Flat regions on these curves indicate a phase transition where all the heat energy provided or removed is used to change the state of the substance, not its temperature. This is where latent heat comes into play.
How do you calculate the heat absorbed or released during phase changes?

+
You can calculate this using Q = mL, where Q is the heat energy, m is the mass, and L is either the latent heat of fusion or vaporization for melting or boiling, respectively.
Can the specific heat capacity be directly read from the curve?

+
No, the specific heat capacity cannot be directly read from the curve. However, the slope of the curve during single-phase regions can give insights into the heat capacity relative to other substances.
How does understanding heating and cooling curves help in real-world applications?

+
By understanding these curves, engineers and scientists can design systems for optimal energy use, predict material behavior, and control industrial processes more effectively.
What is the difference between heat capacity and latent heat?

+
Heat capacity is the amount of heat needed to raise the temperature of a substance by one degree, whereas latent heat is the energy required to change the state of a substance at a constant temperature, without changing its temperature.