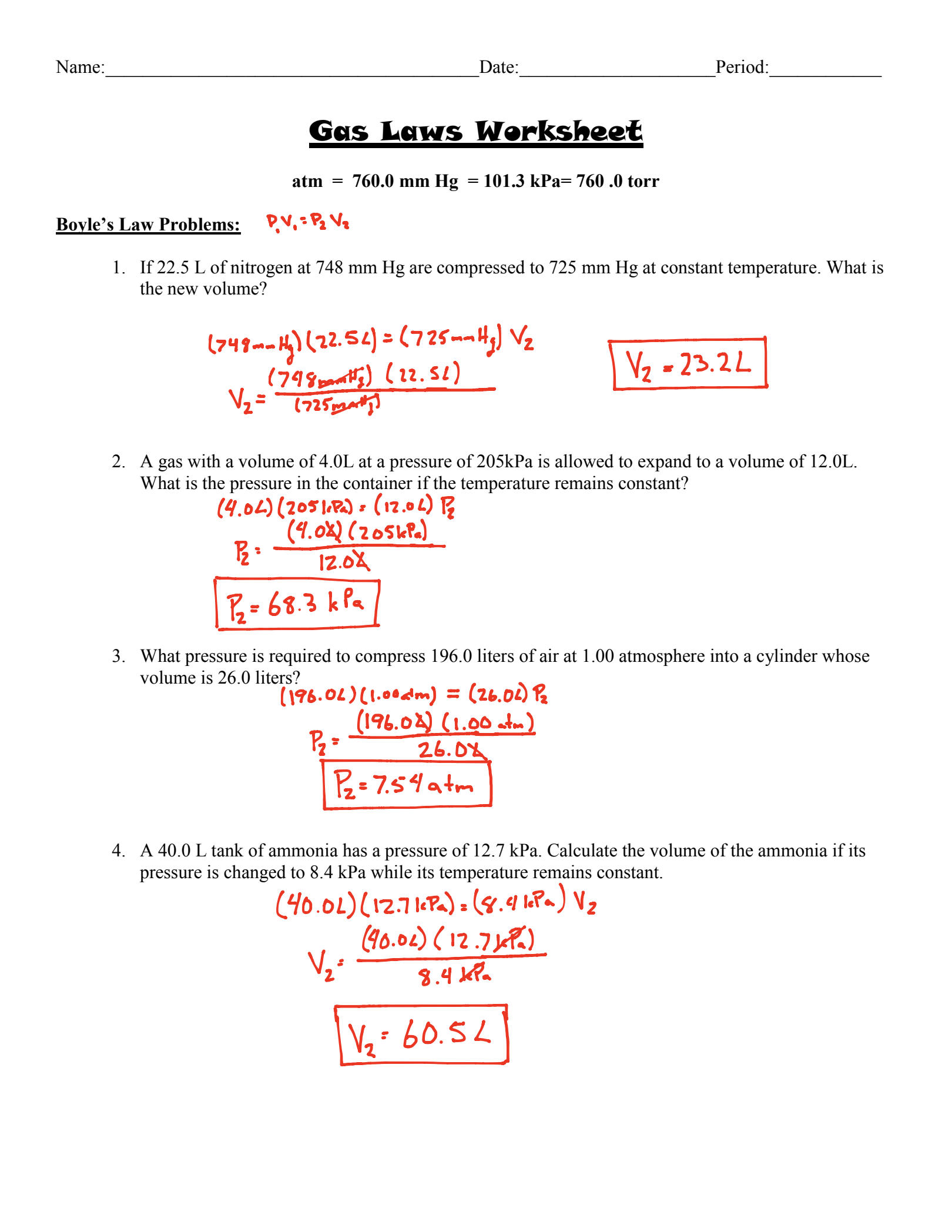
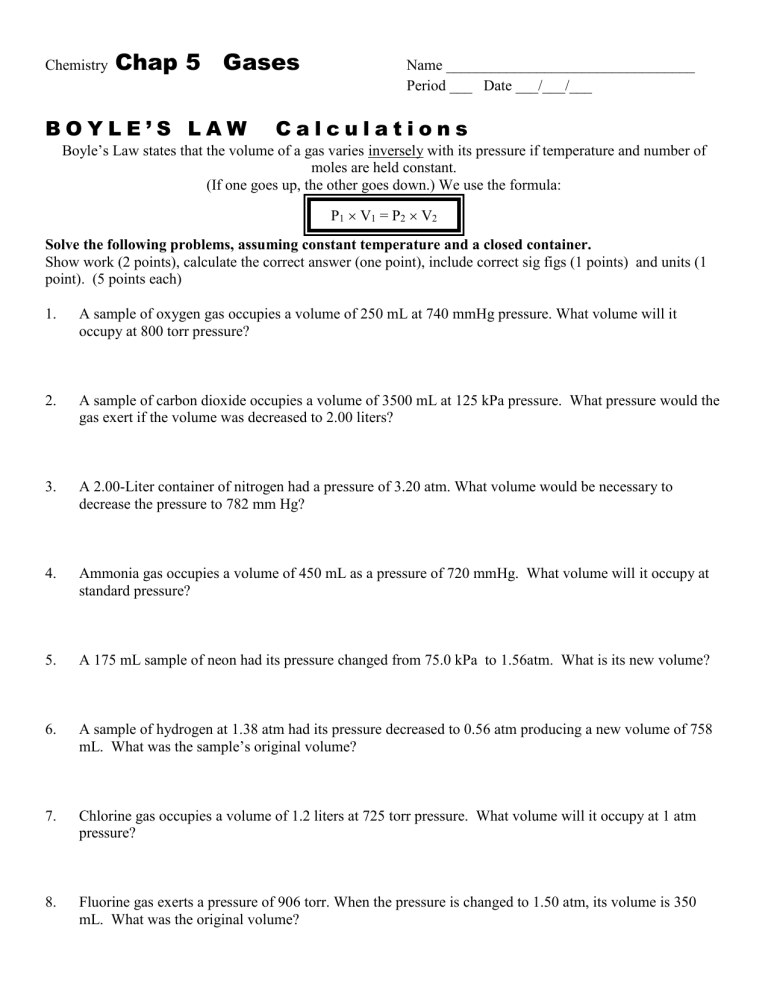
Gas Laws Worksheet 1 Answer Key Unveiled

Understanding gas laws is pivotal for students of chemistry and physics, providing insights into how gases behave under varying conditions. In this comprehensive guide, we delve into the intricacies of the gas laws worksheet 1 by unveiling the answer key, offering step-by-step explanations for each problem, and highlighting common pitfalls students might encounter.
Basic Gas Laws: Understanding the Foundation

At the core of gas behavior, there are three fundamental laws:
- Boyle’s Law: Pressure vs. Volume
- Charles’s Law: Volume vs. Temperature
- Gay-Lussac’s Law: Pressure vs. Temperature
Boyle’s Law: P-V Relationship

Boyle’s Law states that the pressure and volume of a gas have an inverse relationship when temperature is held constant:
P1V1 = P2V2
📚 Note: Ensure to convert units like pressure from atm to mmHg or mL to L as required by the problem.
Charles’s Law: V-T Relationship

Charles’s Law describes how volume changes with temperature at constant pressure:
V1/T1 = V2/T2
Gay-Lussac’s Law: P-T Relationship
Gay-Lussac’s Law states that the pressure of a gas is directly proportional to its temperature at constant volume:
P1/T1 = P2/T2
Combining Gas Laws

These three laws can be combined into a single equation that holds true for any gas, assuming it behaves ideally:
P1V1/T1 = P2V2/T2
Solving Problems Using the Combined Gas Law

When approaching a combined gas law problem, here are the key steps:
- Identify the given values for P, V, and T before and after the change.
- Set up the combined gas law equation.
- Convert units where necessary (e.g., Celsius to Kelvin).
- Plug in the known values, solving for the unknown.
📚 Note: Ensure that all temperatures are in Kelvin (K = °C + 273.15) for accurate calculations.
Sample Problems with Answers

| Problem | Solution |
|---|---|
| A sample of helium gas at 25°C occupies 2.6 L at 1.2 atm pressure. If the temperature is increased to 100°C with the volume unchanged, what will be the new pressure? | Using Gay-Lussac’s Law: P1/T1 = P2/T2 1.2 atm / (25 + 273.15) = P2 / (100 + 273.15) P2 = 1.62 atm |

Further Applications

The principles of gas laws also apply to real-world scenarios like:
- Breathing: How we inhale and exhale
- Aerosol Cans: Pressurized containers and their function
- Weather: Understanding pressure and weather changes
Wrapping Up

Mastering the gas laws is essential not only for academic purposes but for practical understanding of how gases interact in our environment. By understanding the fundamental relationships between pressure, volume, and temperature, students can predict gas behavior under a myriad of conditions. This guide, through its step-by-step approach to solving gas law problems, aims to solidify this understanding, offering a straightforward method to approach these questions confidently.
Why is it important to convert temperature to Kelvin when working with gas laws?
+
Kelvin is used because it starts from absolute zero, which is the lowest possible temperature where molecular motion theoretically stops, making it an ideal scale for gas law calculations.
Can the Ideal Gas Law be used for all gases?

+
The ideal gas law is most accurate for gases at low pressures and high temperatures. However, it can approximate behavior for many real gases but deviates significantly under conditions of high pressure or when the gas molecules are close enough to interact.
What is the significance of Avogadro’s Law in gas behavior?

+
Avogadro’s Law states that equal volumes of all gases, at the same temperature and pressure, have the same number of molecules. This law underpins the relationship between volume and amount (in moles) of gas, providing a way to convert between volume and amount at standard conditions.