5 Easy Steps to Master Polyatomic Ions Formulas

In the world of chemistry, understanding the language of elements and compounds is fundamental. Polyatomic ions play a significant role in this language, often appearing in chemical formulas and reactions. These ions, which consist of more than one atom, carry a charge and are common in both inorganic and organic chemistry. In this post, we'll explore five easy steps to master the formulas of polyatomic ions, ensuring you have the tools to tackle any chemistry challenge with confidence.
Step 1: Familiarization with Common Polyatomic Ions

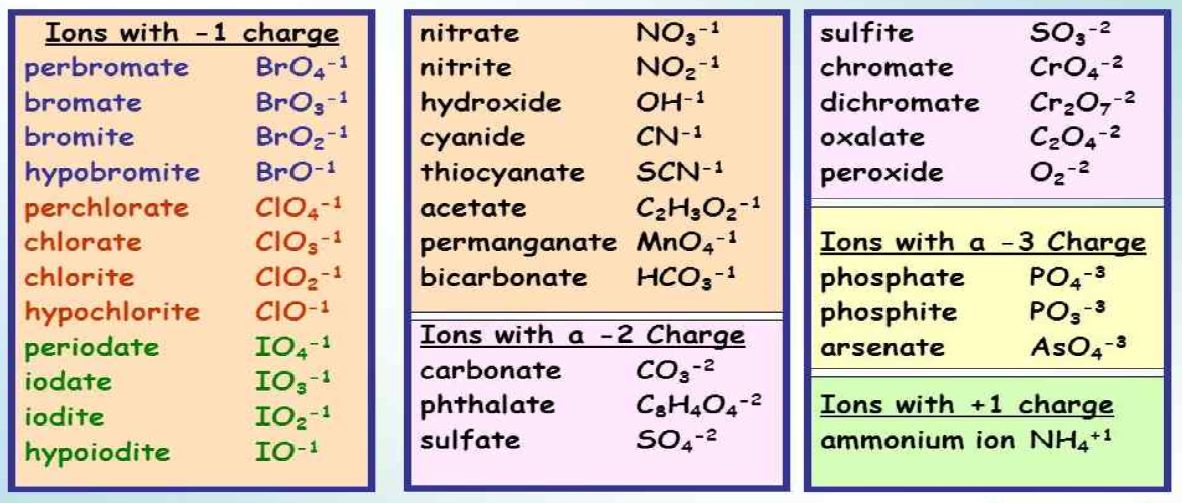
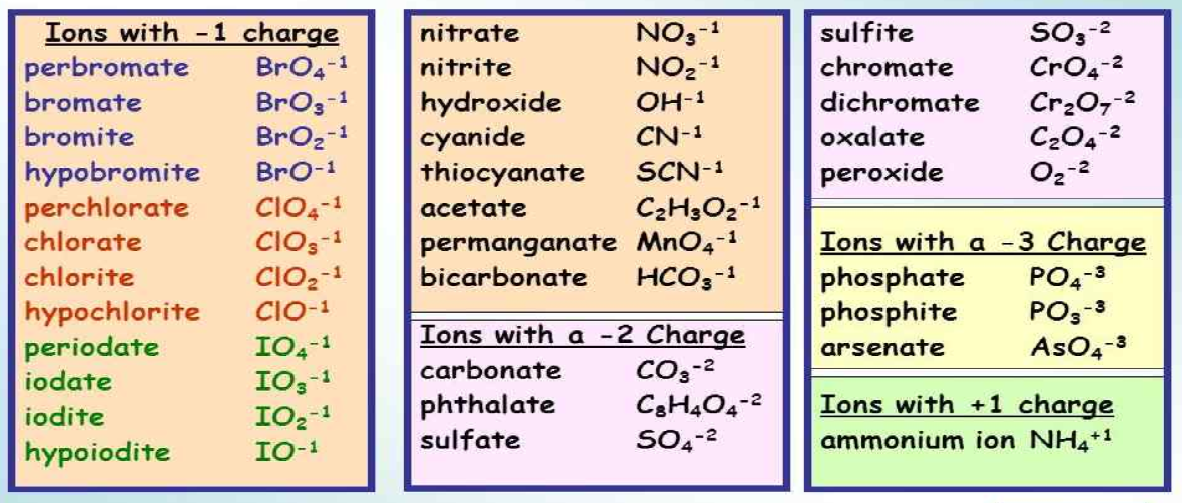
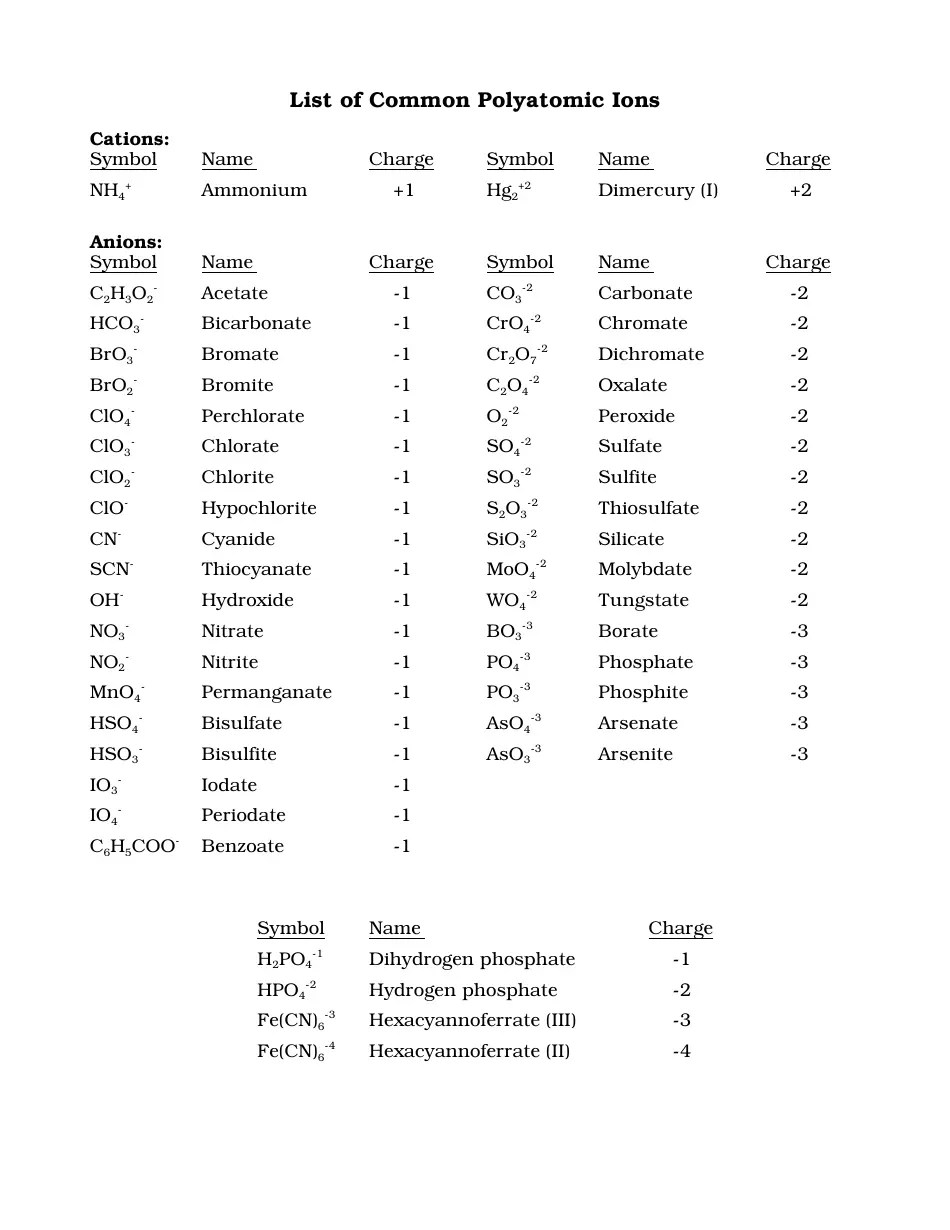
Polyatomic ions come in various forms, each with a unique charge and composition. Here are some of the most common polyatomic ions:
- Nitrate (NO3–)
- Sulfate (SO42–)
- Phosphate (PO43–)
- Hydroxide (OH–)
- Ammonium (NH4+)
📝 Note: Learn the charge of each polyatomic ion. They are consistent, which simplifies the balancing of chemical equations.
Step 2: Understanding Charge Balance
The key to writing correct formulas involving polyatomic ions is balancing the charges. Here’s how you can approach this:
- Identify the charges of all ions involved in the formula.
- Cross over the numerical value of the charge and use it as a subscript for the other ion. If the number is one, do not write it. Simplify if possible.
- Ensure the total positive charges equal the total negative charges.
To illustrate, let’s write the formula for calcium nitrate:
- Calcium ion has a +2 charge (Ca2+).
- Nitrate ion has a -1 charge (NO3–).
- The formula becomes Ca(NO3)2. Calcium crosses over with its +2 charge, becoming NO3, and nitrate crosses over with its -1 charge, becoming Ca1. Simplify to Ca(NO3)2.
Step 3: Recognizing Patterns in Polyatomic Ions

Patterns in polyatomic ions can help you memorize them more easily:
| Polyatomic Ion | Pattern |
|---|---|
| Ammonium | Contains Nitrogen and Hydrogen, forms with +1 charge. |
| Nitrate | Often has three oxygens, with a negative one charge. |
| Hydroxide | Combines Hydrogen and Oxygen with a -1 charge. |

🧠 Note: Recognizing patterns in polyatomic ions can help you guess the formulas of lesser-known ions based on those you know well.
Step 4: Practice Writing Formulas

The more you practice, the better you’ll get. Here are some exercises:
- Write the formula for sodium sulfate.
- Determine the formula of potassium phosphate.
- Calculate the formula of ammonium hydroxide.
Here are the answers:
- Sodium sulfate is Na2SO4. Sodium has a +1 charge, so two sodium ions are needed to balance sulfate’s -2 charge.
- Potassium phosphate is K3PO4. Potassium has a +1 charge, and three potassium ions are needed to balance phosphate’s -3 charge.
- Ammonium hydroxide is NH4OH. Ammonium has a +1 charge, balancing hydroxide’s -1 charge.
💡 Note: Don’t forget to use parenthesis when including more than one polyatomic ion in the formula.
Step 5: Using Mnemonic Devices and Techniques
Mnemonics can be invaluable for memorization:
- SO42–: “Sulfate owes two” for the -2 charge.
- NO3–: “Nitrate knocks one” for the -1 charge.
- PO43–: “Phosphate pays three” for the -3 charge.
By associating charges and ions with phrases or words, you’ll find it easier to recall them during exams or when working out equations.
In summary, mastering the formulas of polyatomic ions involves familiarization with common ions, understanding charge balance, recognizing patterns, practicing writing formulas, and employing mnemonic devices. With these five steps, you'll not only remember polyatomic ion formulas but also grasp their role in chemical reactions, making chemistry not just easier, but also more interesting to study.
What are polyatomic ions?

+
Polyatomic ions are ions that consist of two or more atoms covalently bonded together, carrying a net electric charge.
Why are polyatomic ions important?

+
They are essential in understanding and balancing chemical equations, as they often appear in compounds and play a role in chemical reactions.
How do you balance charges when writing polyatomic ion formulas?

+
The charge of the polyatomic ion is balanced by the charge of the cation or anion involved. Use the cross-over method to determine the correct subscript for each element or ion.
Can you give an example of a mnemonic for a polyatomic ion?
+
For the nitrate ion (NO3–), you could use “Nitrate knocks one” to remember its -1 charge.