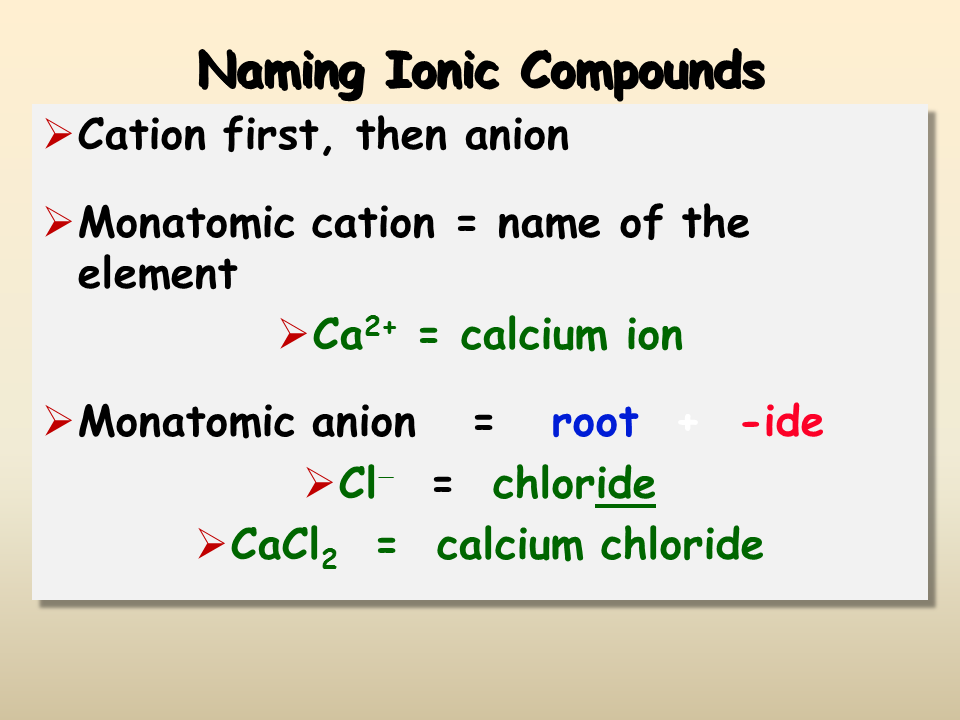
5 Tips for Naming Binary Ionic Compounds Easily

Ionic compounds are the building blocks of chemistry, critical in understanding how elements interact to form compounds. Naming these compounds can sometimes seem like a daunting task, especially for those new to chemistry. However, with a few simple rules and tips, you can master the art of naming binary ionic compounds effectively. In this comprehensive guide, we'll explore five easy strategies to help you name these compounds with confidence.
Tip 1: Understand the Basics

Before diving into the specifics of naming, it’s important to grasp the foundational concepts:
- Binary Compounds: These are compounds that consist of two different elements. An example would be sodium chloride (NaCl).
- Ions: In binary ionic compounds, one element will lose electrons to become positively charged (cation), while the other gains these electrons to become negatively charged (anion).
Remember, cations are named first, followed by the anion. Here are key steps:
- Identify the cation and name it. For metals from group 1 or 2 of the periodic table, this is straightforward as they form only one type of ion.
- For transition metals, which can form multiple ions, a Roman numeral indicating the charge will accompany the metal name.
- Anions are named by replacing the ending of the element’s name with -ide.
Tip 2: Use Periodic Table as Your Tool

The periodic table isn’t just a list of elements; it’s your roadmap to naming compounds:
- Group 1: These metals (like Na, K, Li) form ions with a +1 charge, e.g., sodium (Na+).
- Group 2: Metals here (like Mg, Ca) form ions with a +2 charge, e.g., magnesium (Mg2+).
- Transition Metals: Use the periodic table to determine the charges of transition metals, noting that some might require Roman numerals (Cu can be Cu+ or Cu2+).
- Non-metals: Anions are easily identified by their placement on the periodic table, converting their names to -ide form.
📌 Note: Remember the charge of common ions; for example, oxygen always forms O2- ions, while hydrogen can form H+ or H-.
Tip 3: Master the Roman Numerals

Transition metals present a unique challenge in naming due to their ability to form multiple ions:
- When to use Roman Numerals: If the metal can form multiple ions, use a Roman numeral to indicate the cation’s charge. E.g., FeCl2 is named iron(II) chloride.
- How to Determine Charge: Use the charge of the anion to deduce the charge of the cation, or consult a reference table or periodic table for charges.
| Cation | Charge | Examples |
|---|---|---|
| Cu+ | +1 | CuCl (copper(I) chloride) |
| Cu2+ | +2 | CuSO4 (copper(II) sulfate) |

Tip 4: Recognize Common Polyatomic Ions

Although we are focusing on binary compounds, understanding polyatomic ions can help in more complex compounds:
- Hydroxide: OH- (common in hydroxides like NaOH).
- Nitrate: NO3- (used in compounds like KNO3).
- Phosphate: PO43- (found in compounds like Na3PO4).
These ions have their own names which do not change to -ide, unlike simple anions.
Tip 5: Practice and Memorize

As with any skill, practice makes perfect:
- Create Flashcards: For common ions, names, and charges. Review regularly.
- Name Compounds Regularly: Engage in naming exercises to build your confidence and speed.
- Use Mnemonics: Help remember cation and anion relationships or charges. For example, “sodium sees oxygen off” can help remember sodium loses electrons to oxygen.
Consistency and repetition will help these rules and names become second nature.
By understanding these five tips, naming binary ionic compounds becomes a more manageable task. With the help of the periodic table, a solid grasp on Roman numerals, and an understanding of common ions, you'll be able to navigate the chemical nomenclature with ease. Remember, mastery comes with practice, so keep working on those flashcards, mnemonics, and real-world naming exercises.
Why do some metals require Roman numerals in their names?
+
Transition metals can form multiple ions with different charges. Roman numerals indicate the charge of the metal ion in the compound, thus distinguishing between different forms of the metal (like iron(II) and iron(III)).
How can I remember which elements are anions?

+
Anions are generally non-metals or groups of non-metals found on the right side of the periodic table. Memorize the common anions or use the periodic table to identify their groups, as elements from group 15 to 17 (excluding noble gases) typically form anions.
What happens if I forget the charge of an ion?

+
If you forget the charge of an ion, you can often deduce it from the formula of the compound. Since the sum of charges in a neutral compound must be zero, by knowing the charge of one ion, you can calculate the other’s. Use a periodic table or reference material as a reminder if necessary.