Enzyme Reactions: Simple Worksheet to Understand Biochemistry Basics

Understanding enzyme reactions is fundamental for anyone delving into the world of biochemistry. Enzymes, often referred to as biological catalysts, play a crucial role in speeding up chemical reactions essential for life processes. This post will guide you through the basics of enzyme reactions using a simple worksheet approach, which will help demystify how these tiny molecules control our metabolic pathways.
What Are Enzymes?

Enzymes are proteins that increase the rate of chemical reactions within the body. They are not consumed in these reactions but merely aid in lowering the activation energy required for the reactions to occur. Here are some key points about enzymes:
- They act by providing an alternative reaction pathway with a lower activation energy.
- They are specific in their substrate binding due to their active site's shape.
- Enzymes can be affected by environmental factors like temperature, pH, and substrate concentration.
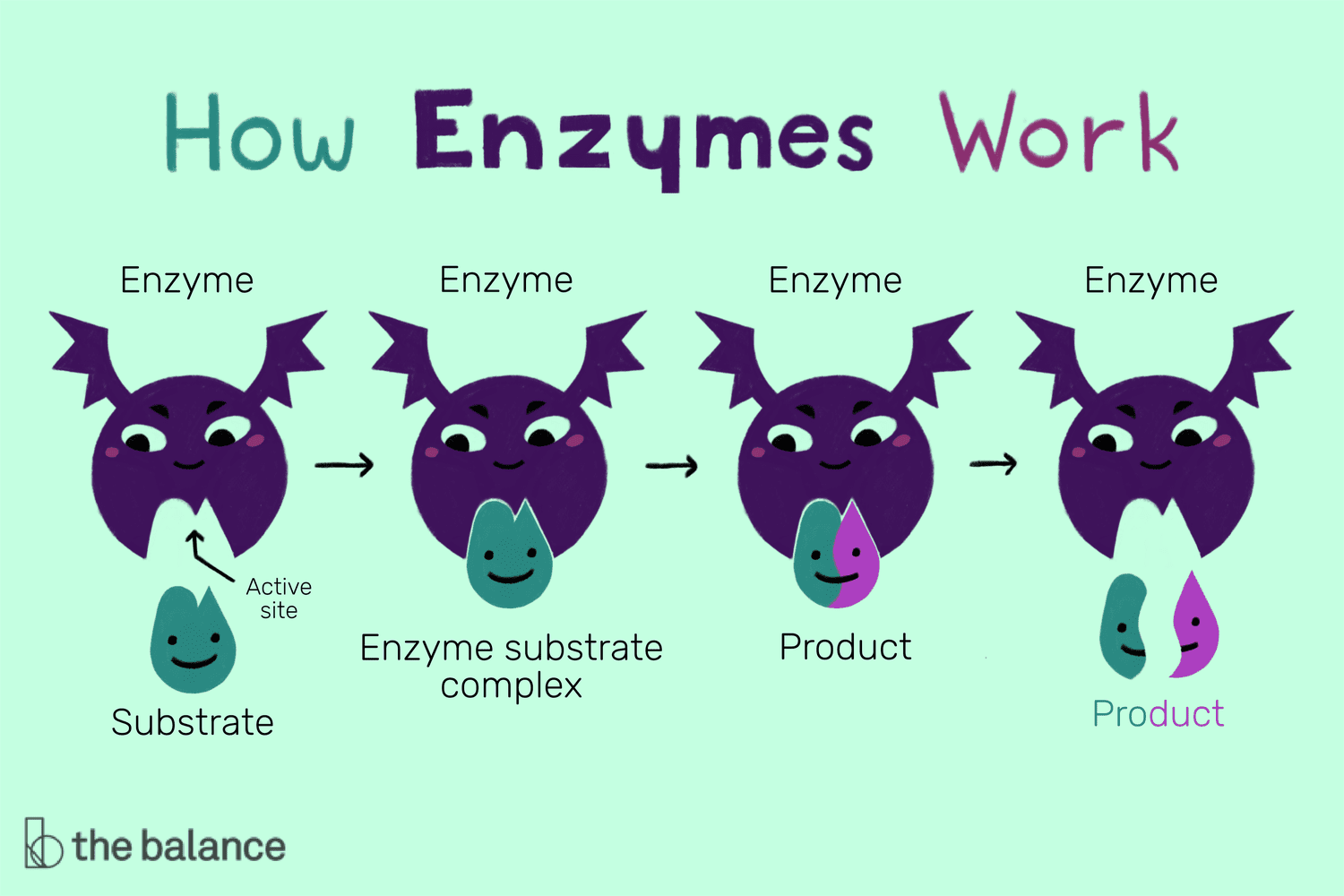
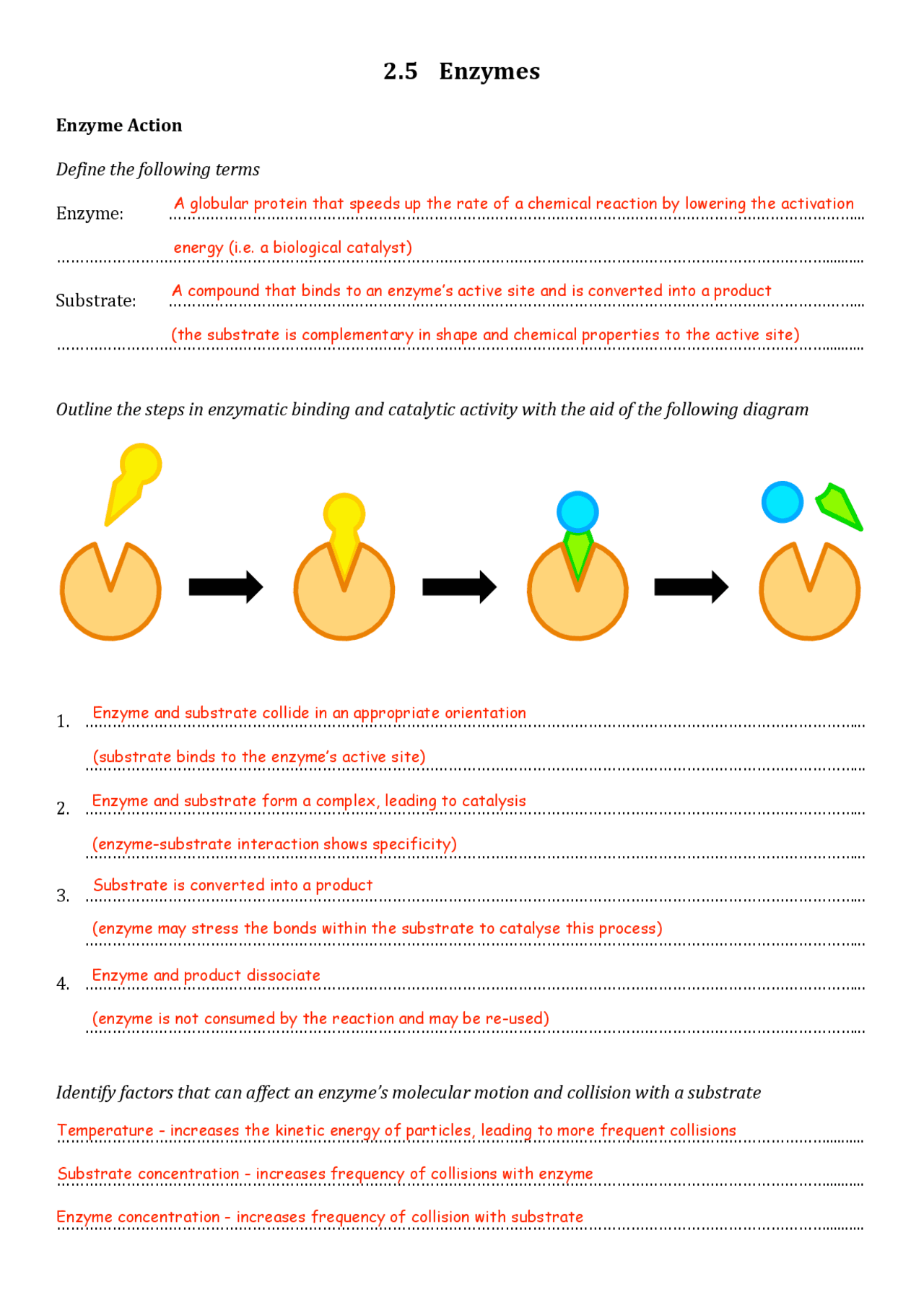
Enzyme Reaction Mechanism

To understand how enzymes work, let's walk through the mechanism using a hypothetical reaction:
| Step | Description |
|---|---|
| 1. Substrate Binding | The enzyme forms an enzyme-substrate complex by binding with the substrate at its active site. |
| 2. Transition State Formation | The enzyme induces strain on the substrate, pushing it towards a transition state, which is easier to convert to product. |
| 3. Catalysis | The enzyme lowers the activation energy, allowing the substrate to change into the product. |
| 4. Product Release | Once the reaction is complete, the product is released, freeing up the enzyme to catalyze another reaction. |

🌿 Note: Enzyme reactions are reversible, meaning that under certain conditions, the products can revert back to substrates.
Factors Affecting Enzyme Activity
Enzyme activity isn't constant and can be influenced by several environmental factors:
- Temperature: Enzymes have an optimal temperature at which they function best. Deviations can denature enzymes or slow down their activity.
- pH: Each enzyme has an optimal pH range where its activity is maximized.
- Substrate Concentration: More substrate increases the reaction rate until saturation, where all enzyme active sites are occupied.
- Enzyme Concentration: Increasing the amount of enzyme increases the rate of reaction until the substrate concentration becomes limiting.
- Inhibitors and Activators: Certain molecules can inhibit or enhance enzyme activity.
Worksheet: Understanding Enzyme Reactions
To better grasp the concepts, here’s a simple worksheet to work through:
- List three ways enzymes speed up reactions.
- Describe the "lock and key" and "induced fit" models of enzyme-substrate interactions.
- How does an enzyme's optimal pH influence its activity?
- What would happen if an enzyme's active site was altered?
Each of these questions aims to reinforce your understanding through active learning.
✏️ Note: When filling out worksheets like this, take time to think about real-life examples or analogies that can help explain these biochemical principles.
This dive into enzyme reactions illustrates how these proteins are critical to maintaining life at the cellular level. Understanding their mechanism, specificity, and environmental sensitivities not only enriches our knowledge of biochemistry but also highlights their importance in medicine, industry, and environmental management. Enzyme reactions are at the heart of our metabolic processes, and a deep understanding of these tiny catalysts allows us to better comprehend and manipulate life's intricate biochemical pathways.
What’s the difference between an enzyme and a catalyst?

+
An enzyme is a biological catalyst, which means it’s a catalyst produced by living organisms to speed up biological reactions. Catalysts in general can be inorganic, like metals or acids, while enzymes are specifically proteins or RNA molecules with catalytic activity.
How can environmental conditions impact enzyme function?

+
Enzyme activity can be altered by changes in temperature, pH, substrate concentration, and the presence of inhibitors or activators. These conditions affect the enzyme’s ability to bind with its substrate effectively.
Why are enzymes reusable?

+
Enzymes are not consumed in the reactions they catalyze. After the reaction, the enzyme is released in its original form, allowing it to catalyze additional reactions without further synthesis.
Can enzymes be repaired if they’re damaged?

+
Yes, some enzymes can undergo repair mechanisms within cells. For example, when DNA repair enzymes are damaged, the cell has mechanisms to either replace or repair these enzymes.