Energy Diagram Worksheet Answers: Your Complete Guide

If you've ever found yourself staring blankly at an energy diagram worksheet, you're not alone. Energy diagrams, also known as reaction coordinate diagrams, can appear daunting at first glance, but they are actually quite straightforward once you understand their components and how to interpret them. Whether you're a student grappling with chemistry or physics, or simply someone with an interest in understanding how energy changes in chemical reactions, this guide will provide you with a comprehensive understanding of energy diagrams.
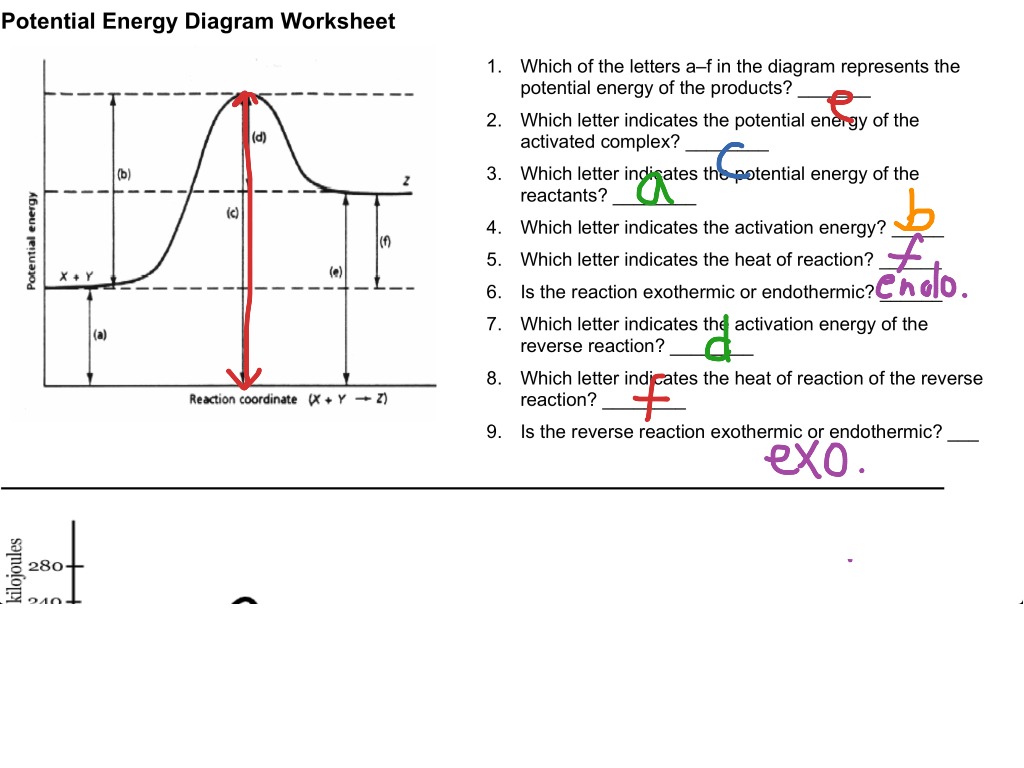
What are Energy Diagrams?

Energy diagrams illustrate how energy changes throughout the course of a chemical reaction. Here’s what you need to know:
- Reaction Coordinate: This is the horizontal axis, representing the progress of the reaction from reactants to products.
- Potential Energy: The vertical axis shows the energy of the reactants, intermediates, and products.
- Reactants and Products: The starting and ending points, where the reactants are at the beginning and products at the end of the reaction.
- Energy Barrier or Activation Energy (Ea): This is the minimum energy needed for the reaction to occur, depicted as a hill or peak on the graph.
- Transition State: This is the highest energy point on the diagram, where bonds are in the process of breaking and forming.
- Enthalpy Change (ΔH): The difference in energy between reactants and products, showing if the reaction is exothermic (energy release, ΔH < 0) or endothermic (energy absorption, ΔH > 0).
Understanding Exothermic vs. Endothermic Reactions

Two key types of reactions are represented by energy diagrams:
- Exothermic Reactions: Here, the energy of the products is lower than that of the reactants. The ΔH is negative because energy is released during the reaction. The diagram shows a descent from reactants to products.
- Endothermic Reactions: In contrast, the energy of the products is higher than that of the reactants. The ΔH is positive as energy is absorbed from the environment. The graph shows an ascent from reactants to products.
Analyzing Energy Diagrams

When you are analyzing an energy diagram:
- Identify Reactants and Products: Look for where the energy starts and ends on the vertical axis.
- Find Activation Energy (Ea): This is the energy difference from the reactants to the transition state.
- Determine the Type of Reaction: Check if ΔH is negative or positive to identify if it’s an exothermic or endothermic reaction.
- Observe Reaction Intermediates: Some reactions might show multiple peaks or plateaus representing intermediates.
Example Worksheet Question

Here’s how you might answer a common question on an energy diagram worksheet:
Question:

Given an exothermic reaction, identify the following:
- The reactants and products
- The activation energy
- The ΔH of the reaction
Answer:

Using the energy diagram:
- Reactants: Start with a higher energy state.
- Products: End with a lower energy state than reactants.
- Activation Energy (Ea): Calculate the difference in energy from the reactants to the peak (transition state).
- ΔH: Measure the vertical difference from the energy of reactants to products. In an exothermic reaction, this value will be negative.
⚠️ Note: Ensure the energy units are consistent throughout your analysis (e.g., joules or kilojoules).
By interpreting the diagram correctly, you can answer various questions related to the reaction's kinetics and thermodynamics. Understanding the energy landscape gives insights into the reaction's efficiency and the conditions needed for the reaction to proceed.
FAQ Section

What does it mean if ΔH is positive?

+
A positive ΔH indicates an endothermic reaction where the products have more energy than the reactants, meaning energy is absorbed from the surroundings.
How do catalysts affect energy diagrams?
+
Catalysts lower the activation energy (Ea) by providing an alternative pathway for the reaction, which results in a lower peak in the energy diagram, but does not change the ΔH.
Why does the transition state have the highest energy?

+
The transition state is where bonds are breaking and forming, requiring the highest energy input because the system is at its most unstable form before the reaction proceeds to form products.
Can a reaction proceed if it does not have enough energy?

+
If a reaction does not have sufficient energy to overcome the activation energy barrier, it will not proceed; however, catalysts can lower this barrier allowing the reaction to occur at a lower energy threshold.
In summary, understanding energy diagrams opens up a window into the energetic world of chemical reactions. They provide a visual representation of how energy is transformed during a reaction, guiding us in predicting how reactions behave under various conditions. Through practice with energy diagram worksheets, you’ll gain proficiency in interpreting these diagrams, thereby mastering the kinetics and thermodynamics of chemical reactions. This knowledge isn’t just crucial for academic purposes but also has practical applications in fields like pharmaceuticals, material science, and beyond where controlling reactions is paramount.