Emission Spectra Worksheet Answers: Simplifying Energy Levels

Understanding the concept of emission spectra is crucial in grasping how elements interact with light and energy, forming the basis for many spectroscopic analyses in both theoretical and applied sciences. Emission spectra provide a fingerprint-like pattern unique to each element, allowing scientists to identify and quantify elemental compositions in a variety of samples, from the stars above to the minerals on Earth. In this blog post, we delve into emission spectra, offering a comprehensive guide to what they are, how they work, and how you can navigate through worksheet answers related to this subject.
What Are Emission Spectra?

An emission spectrum is the range of electromagnetic radiation that is emitted from an element or compound when it transitions from an excited state to a lower energy state. Here’s how it works:
- Energy Absorption: An atom or molecule absorbs energy, often in the form of light or heat, which promotes electrons to higher energy levels.
- Energy Emission: When these electrons return to their ground state or lower energy levels, they release the absorbed energy as photons. The energy of these photons is directly related to the difference in energy levels, giving us characteristic wavelengths of light for each element.
Understanding Energy Levels
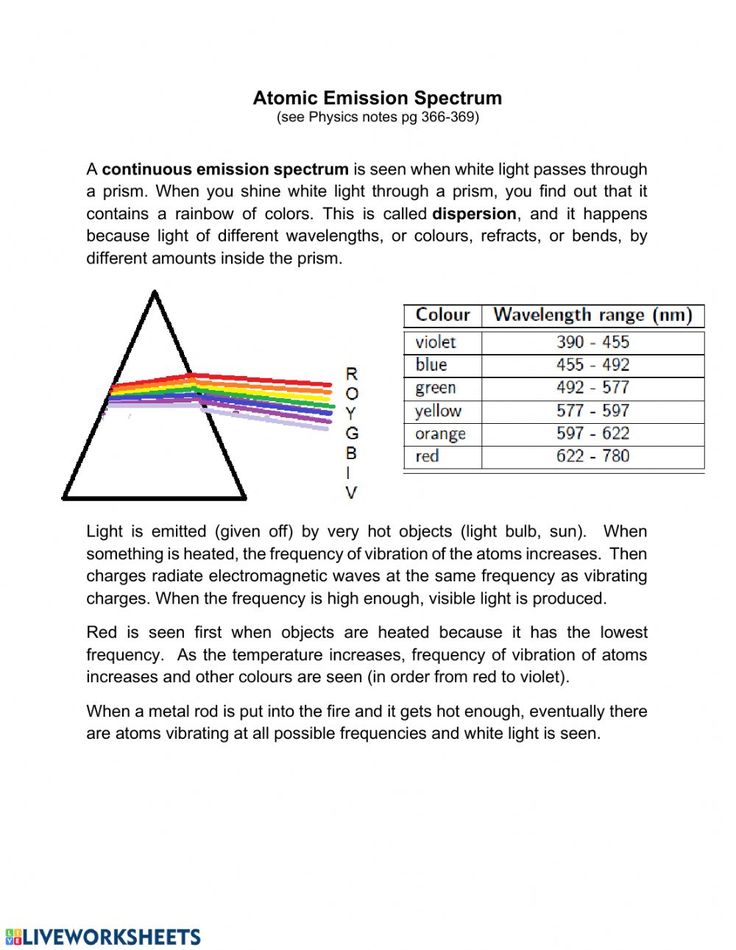
Energy levels, often depicted in diagrams, are quantized and discrete for atoms and molecules:
- Ground State: The lowest energy level, where electrons typically reside.
- Excited States: Higher energy levels where electrons move to upon energy absorption.
- Transitions: When electrons move from one energy level to another, they emit or absorb specific wavelengths of light.
⚡ Note: The transitions are governed by the laws of quantum mechanics, ensuring that only specific transitions are possible, which is why each element has a unique emission spectrum.
Emission Spectra Worksheet Answers
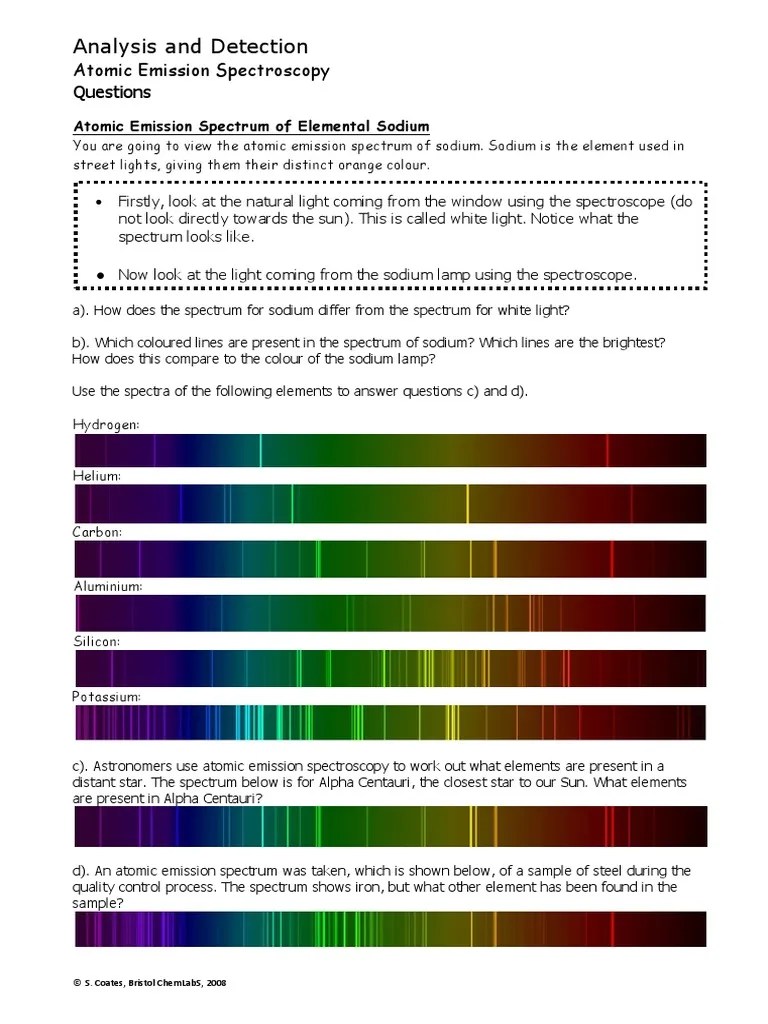
Types of Emission Spectra

Emission spectra can be categorized as follows:
- Continuous Spectrum: Produced by hot, dense objects like black bodies, where the emitted light covers all wavelengths.
- Emission Line Spectrum: When electrons in low-pressure gases fall back to lower energy levels, this results in discrete lines on the spectrum.
- Absorption Spectrum: Although not an emission, it’s worth mentioning as it’s the opposite, where continuous light passes through a cooler gas, and certain wavelengths are absorbed.
Interpreting Emission Spectra

Here’s how to interpret emission line spectra in a worksheet:
- Element Identification: Each element has its unique set of lines. By comparing observed spectra to known spectra, you can identify the elements present.
- Energy Transitions: The position of lines on the spectrum indicates the energy difference between electron energy levels.
- Temperature Determination: Line width and intensity can indicate the temperature of the source; broader lines suggest higher temperatures due to Doppler broadening.
Calculation of Wavelengths

Worksheets might ask you to calculate the wavelengths of emission lines. Here’s how:
- Identify the transition from a higher to a lower energy level.
- Use the equation E = hc/λ where:
- E is the energy difference (in Joules).
- h is Planck’s constant.
- c is the speed of light in a vacuum.
- λ is the wavelength of emitted light.
- Solve for λ.
Applications of Emission Spectra

Emission spectra are utilized in:
- Astronomy: Analyzing the composition of stars and interstellar medium.
- Chemistry: Identifying and quantifying elements in samples.
- Physics: Studying the behavior of atoms and molecules under various conditions.
In wrapping up our exploration of emission spectra, we've covered their fundamental nature, the mechanics of energy transitions, and practical applications in various fields. This knowledge not only enhances our understanding of the universe but also equips scientists and students with tools for elemental analysis. Remember, when you're working through emission spectra worksheets or problems:
💡 Note: Pay attention to the specific transitions described, as each transition corresponds to a distinct wavelength of light emitted.
What does a continuous spectrum indicate?

+
A continuous spectrum indicates that the source is emitting light at all wavelengths within a given range, typically seen in incandescent solids, liquids, or dense gases.
Can an element emit multiple wavelengths?

+
Yes, an element can emit multiple wavelengths as electrons can transition between various energy levels, each transition emitting light at a specific wavelength.
How can you distinguish between emission and absorption spectra?

+
Emission spectra show bright lines against a dark background, whereas absorption spectra show dark lines against a continuous spectrum where certain wavelengths have been absorbed.



