5 Easy Ways to Distinguish Elements, Compounds, Mixtures

Understanding the fundamental concepts of matter is essential for anyone delving into the world of chemistry, physics, or material science. The distinctions between elements, compounds, and mixtures not only underpin basic chemical theory but also play a crucial role in industrial processes, environmental science, and daily life applications. This post explores five straightforward ways to differentiate these basic forms of matter, making it easier for students, hobbyists, and professionals to categorize substances accurately.
The Basics of Matter

Before we dive into the methods of distinguishing elements, compounds, and mixtures, let’s briefly review what these terms mean:
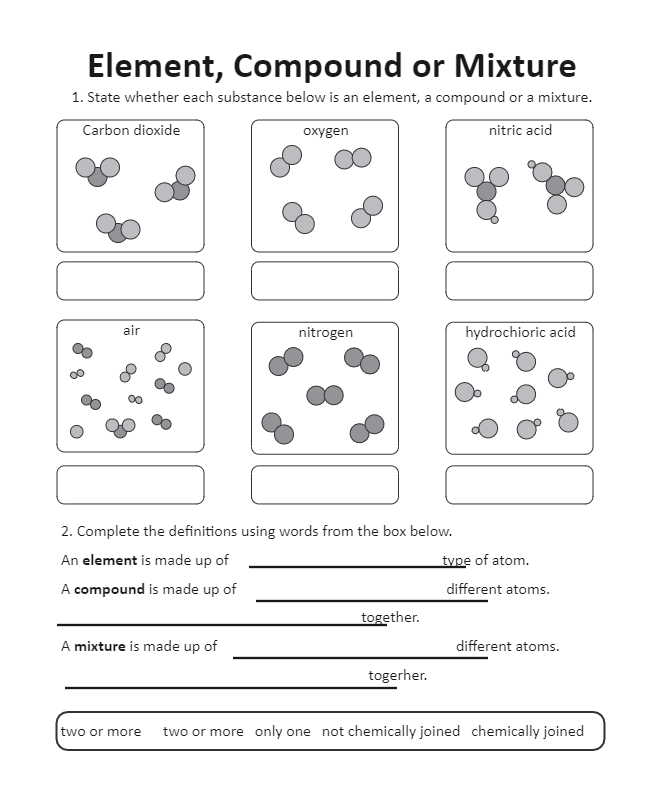
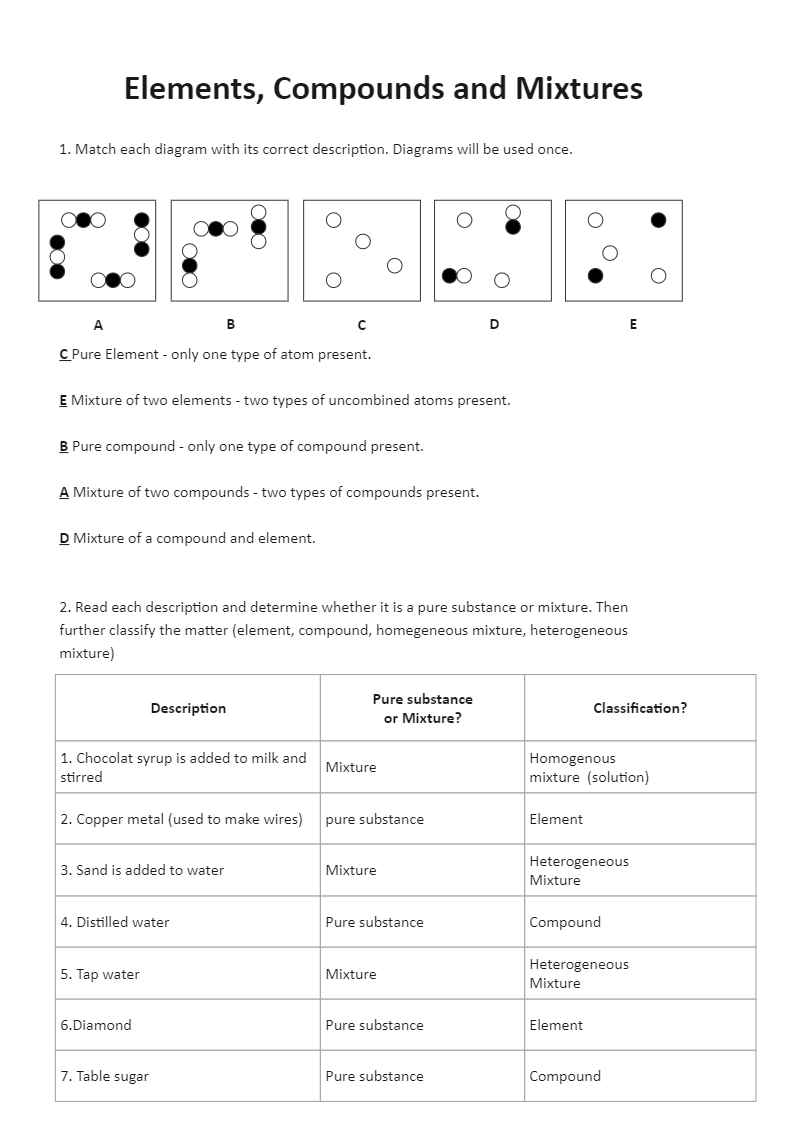
- Elements: Pure substances that cannot be broken down into simpler substances by chemical reactions. They consist of only one type of atom. Examples include oxygen, hydrogen, and gold.
- Compounds: Substances formed when two or more elements chemically combine. They have a fixed composition and can be broken down into their constituent elements. Water (H2O) is a classic example, composed of hydrogen and oxygen.
- Mixtures: Physical combinations of two or more substances where each maintains its chemical identity. Mixtures can be homogeneous, like salt water, or heterogeneous, like granola cereal.
1. Analytical Techniques

One of the most scientific and precise methods to identify substances:
- Spectroscopy: This method uses light to analyze the composition of matter. Techniques like mass spectrometry or atomic emission spectroscopy can distinguish elements and compounds based on their atomic or molecular signatures.
- Chromatography: This technique separates mixtures by passing them through a medium where different components travel at different speeds. Gas chromatography and liquid chromatography are common in labs to distinguish between complex mixtures.
🔬 Note: Advanced analytical techniques require specialized equipment and trained personnel. They are often used in research settings but can be accessed by educational institutions.
2. Chemical Reactions
Chemical reactions can help differentiate:
- Decomposition: Compounds can be broken down into their constituent elements through heat or electrolysis. If a substance remains unchanged after applying such conditions, it’s likely an element or a mixture.
- Reactivity Tests: Elements often react in predictable ways. For example, metals react with acids to produce hydrogen gas, indicating the presence of elements like magnesium or zinc.
3. Physical Properties

Simple observations can sometimes suffice:
- Appearance: While not definitive, elements often have a characteristic look (e.g., gold’s yellow hue, oxygen’s colorless and odorless nature). Compounds might change color or appearance under certain conditions.
- Density and Melting Points: Pure elements and compounds have distinct, measurable physical properties. Mixtures’ properties can vary widely.
4. Melting and Boiling Points

Temperature changes provide clear indicators:
- Fixed Points: Elements and compounds have a specific melting or boiling point at which the entire substance changes phase. Mixtures, however, melt or boil over a range of temperatures.
- Behavior Under Heat: Observing how a substance behaves when heated can give clues. If it melts at a single temperature, it’s likely an element or compound; if it doesn’t or melts over a range, consider it might be a mixture.
5. Separation Techniques

Various physical methods can separate mixtures:
- Filtration: If a substance has both solid and liquid components, filtration can separate them, indicating a mixture.
- Evaporation: Heating a mixture can evaporate the more volatile component, leaving behind the less volatile substance.
- Magnetism: Using a magnet can separate magnetic from non-magnetic components, a clear sign of a mixture.
💡 Note: These methods are especially useful in educational settings where understanding the principles of separation can illustrate the differences between substances.
In exploring these methods for distinguishing elements, compounds, and mixtures, we’ve covered a broad spectrum of techniques that cater to different levels of analysis. From basic observations to sophisticated laboratory techniques, each method provides valuable insights into the nature of substances. This knowledge isn’t just theoretical; it has practical applications in industry for quality control, in environmental sciences for identifying pollutants, and in daily life for understanding the materials around us. By recognizing how these substances differ, we not only grasp the complexity of the world we live in but also gain tools to interact with and manipulate our environment in meaningful ways.
Why do elements have fixed properties while mixtures do not?

+
Elements consist of only one type of atom, so they have fixed physical and chemical properties. Mixtures, however, contain multiple substances, each retaining their own properties, leading to variable overall properties.
Can mixtures be separated into elements and compounds?

+
Yes, mixtures can be separated using physical methods like filtration or distillation. However, compounds within the mixture must be chemically broken down to return to their elemental forms.
Is air an element, compound, or mixture?

+
Air is a mixture of several gases, primarily nitrogen (78.09%) and oxygen (20.95%), along with smaller amounts of other gases like argon, carbon dioxide, and neon.
How can a compound be identified?
+
Compounds can be identified through their fixed composition, unique chemical and physical properties, and by observing their behavior in chemical reactions or during changes in temperature (like melting or boiling points).