5 Essential Answers for Compounds and Mixtures Worksheet

Understanding the differences between compounds and mixtures is fundamental in chemistry. A compound worksheet, along with a mixtures worksheet, often poses some challenging questions for students. Here, we will tackle five essential questions commonly found in such worksheets, providing clear, step-by-step answers to enhance your comprehension of these chemical classifications.
The Composition of Compounds and Mixtures


Compounds and mixtures differ fundamentally in their composition:
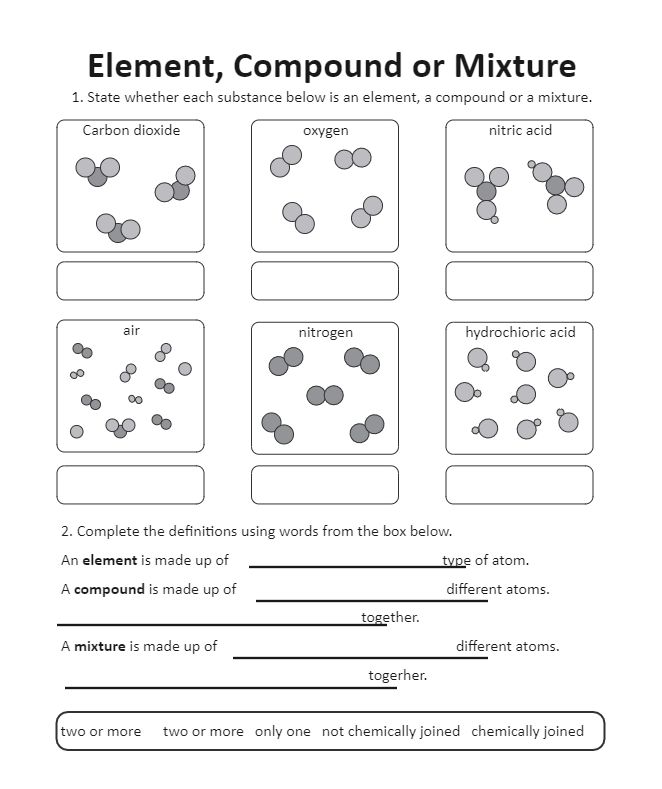
- Compounds: Formed when atoms of different elements chemically combine in fixed ratios. These bonds create new substances with unique properties, distinct from the properties of their constituent elements.
- Mixtures: Composed of two or more substances physically mixed together in variable proportions. The substances in a mixture retain their individual properties and can be separated by physical methods.
Separation Techniques for Mixtures

Mixtures can be separated using various physical methods. Here are some common techniques:
- Filtration: Effective for separating solid particles from liquids or gases. A fine mesh or filter paper traps the solid, allowing the liquid or gas to pass through.
- Distillation: Utilizes differences in boiling points to separate components. As the mixture is heated, each substance vaporizes at different temperatures, allowing for collection of the vapors individually.
- Chromatography: Various types (paper, thin-layer, column, gas, etc.) enable the separation of complex mixtures by exploiting different affinities of compounds for a mobile and stationary phase.
Physical Properties of Mixtures

While mixtures do not have a single set of chemical properties, their physical properties can be measured:
- Density: The mass of a mixture divided by its volume.
- Viscosity: Resistance to flow; varies with the composition of the mixture.
- Boiling and Melting Points: These points depend on the individual components’ melting and boiling points, unlike in compounds where these are fixed.
- Conductivity: Can change based on the presence of ionic substances within the mixture.
Formation of Compounds

Compounds are formed through chemical reactions where reactants transform into new substances with distinct characteristics:
- Combination Reactions: Two or more elements or compounds combine to form a single compound.
- Decomposition Reactions: A compound breaks down into its constituent elements or simpler compounds.
- Combustion Reactions: A substance reacts with oxygen, often producing heat and light, to form an oxide.
🔍 Note: Chemical reactions always obey the law of conservation of mass, meaning the total mass of reactants equals the total mass of products.
Practical Applications

The understanding of compounds and mixtures extends beyond academic exercises:
- Pollution Control: Techniques like distillation are used to separate pollutants from water.
- Pharmaceuticals: Separation and purification of compounds are crucial in drug development.
- Materials Science: Mixtures are engineered for specific physical properties in industries like construction or electronics.
- Environmental Chemistry: Identifying and separating mixtures aids in studying environmental impact.
In summary, recognizing the distinctions between compounds and mixtures is crucial for not only understanding chemistry but also for practical applications across various fields. The differences in how they are formed, their properties, and the methods to separate them are essential for solving real-world problems and advancing technological development.
What is the primary difference between a mixture and a compound?

+
The primary difference is that compounds are chemically bonded, creating substances with new properties, while mixtures retain the properties of their individual components and are not chemically bonded.
Can a mixture have a definite formula?
+
No, mixtures do not have a definite formula because their composition can vary. Compounds, on the other hand, have fixed chemical formulas.
How do you separate a mixture that contains salt and sand?

+
You can separate salt from sand using filtration followed by evaporation. First, dissolve the salt in water, filter out the sand, then evaporate the water to recover the salt.