5 Essential Elements Compounds and Mixtures Review
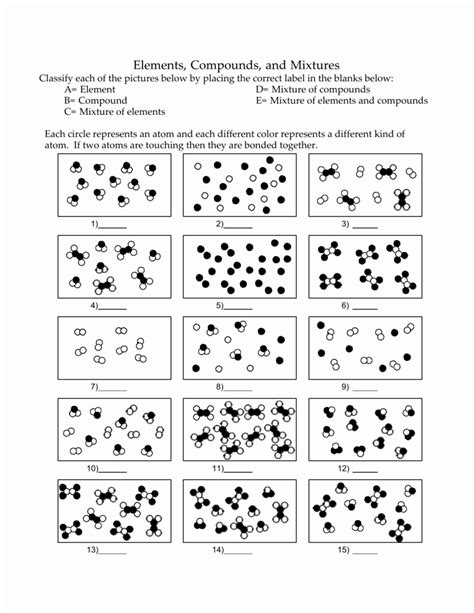
Understanding the Building Blocks of Matter: Elements, Compounds, and Mixtures

The study of chemistry is deeply rooted in the understanding of elements, compounds, and mixtures. These three concepts are fundamental to understanding the composition of matter and how it interacts with other substances. In this review, we will delve into the definitions, characteristics, and differences between elements, compounds, and mixtures, as well as explore their importance in various fields of science.
Elements: The Foundation of Matter

Elements are the simplest substances in chemistry, consisting of only one type of atom. They are the building blocks of matter and cannot be broken down into simpler substances by chemical means. Elements are characterized by their unique atomic number, which is the number of protons present in the nucleus of an atom. There are 118 known elements, and each has its own distinct properties and characteristics.
Some key features of elements include:
- Atomicity: Elements are composed of only one type of atom.
- Uniqueness: Each element has a unique atomic number and set of properties.
- Indivisibility: Elements cannot be broken down into simpler substances by chemical means.
Examples of elements include hydrogen (H), oxygen (O), and carbon ©.
Compounds: The Combination of Elements
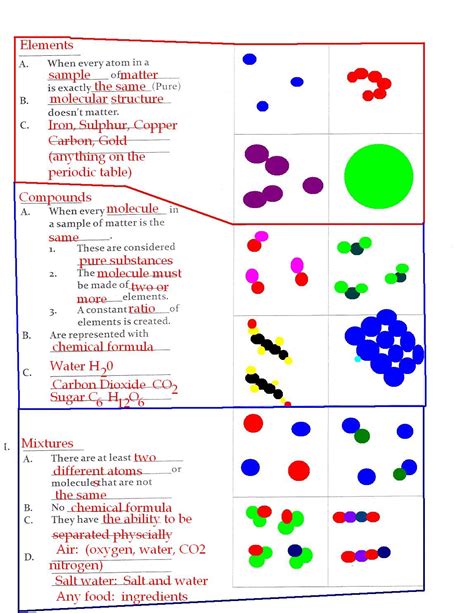
Compounds are substances formed when two or more different elements are chemically bonded together. The resulting compound has properties that are distinct from those of the individual elements. Compounds can be either molecular, meaning they consist of molecules, or ionic, meaning they consist of ions.
Some key features of compounds include:
- Composition: Compounds are composed of two or more different elements.
- Chemical bonding: Elements are chemically bonded together to form a compound.
- New properties: Compounds have properties that are distinct from those of the individual elements.
Examples of compounds include water (H2O), carbon dioxide (CO2), and ammonia (NH3).
💡 Note: Compounds can be further classified into molecular compounds and ionic compounds, each with its own set of characteristics and properties.
Mixtures: The Combination of Substances

Mixtures are physical combinations of two or more substances, which can be either elements or compounds. Mixtures do not involve chemical bonding between the components, and the resulting mixture has properties that are a combination of the individual substances.
Some key features of mixtures include:
- Composition: Mixtures are composed of two or more substances.
- Physical combination: Substances are physically combined, without chemical bonding.
- Variable properties: Mixtures have properties that are a combination of the individual substances.
Examples of mixtures include air (a mixture of gases), seawater (a mixture of water and various salts), and soil (a mixture of minerals, organic matter, and living organisms).
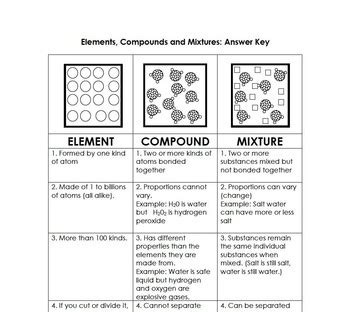
| Element | Compound | Mixture |
|---|---|---|
| One type of atom | Two or more different elements | Two or more substances |
| Unique atomic number | Chemical bonding between elements | Physical combination of substances |
| Indivisible by chemical means | New properties distinct from elements | Properties a combination of individual substances |
Importance of Elements, Compounds, and Mixtures

Understanding the differences between elements, compounds, and mixtures is crucial in various fields of science, including chemistry, biology, physics, and engineering. The ability to identify and distinguish between these substances is essential for:
- Chemical synthesis: Understanding the composition and properties of elements and compounds is critical for synthesizing new substances.
- Materials science: The study of compounds and mixtures is essential for developing new materials with unique properties.
- Environmental science: Understanding the composition and properties of mixtures is crucial for monitoring and mitigating environmental pollution.
As we continue to explore and discover new substances, the distinction between elements, compounds, and mixtures becomes increasingly important.
In conclusion, the understanding of elements, compounds, and mixtures is fundamental to the study of chemistry and other sciences. By recognizing the unique characteristics and properties of each, we can better comprehend the composition of matter and develop new substances with unique properties.
What is the main difference between an element and a compound?

+
The main difference between an element and a compound is that an element consists of only one type of atom, while a compound is composed of two or more different elements chemically bonded together.
Can a mixture be composed of only one type of substance?

+
No, a mixture by definition is composed of two or more substances. If a substance is composed of only one type of substance, it is considered a pure substance, not a mixture.
What is the importance of understanding the difference between elements, compounds, and mixtures in chemistry?

+
Understanding the difference between elements, compounds, and mixtures is crucial in chemistry as it allows us to identify and distinguish between substances, which is essential for chemical synthesis, materials science, and environmental science.
Related Terms:
- element compound mixture worksheet pdf
- substances vs mixtures worksheet answers
- identifying elements compounds and mixtures
- elements vs compounds worksheet pdf
- identifying elements and compounds worksheet
- elements vs compounds mixtures worksheet



