5 Tips for Solving Electrons in Atoms Worksheets

Electron configurations and the understanding of atomic structure are foundational skills in the world of chemistry, essential for students studying both at high school and university levels. When you're tasked with completing electrons in atoms worksheets, it can often seem daunting to get all the details correct. To aid you in mastering this concept, here are five essential tips that will not only help you solve these worksheets but also deepen your understanding of atomic electron configurations.
1. Understand the Aufbau Principle
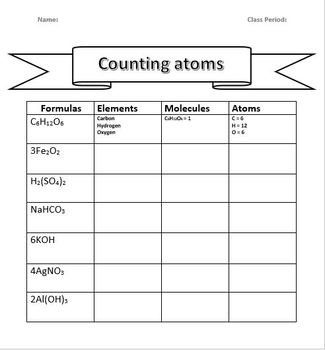
The Aufbau principle states that electrons occupy the lowest energy orbitals available. Here’s how you can apply this principle:
- Start filling electrons from the lowest energy level to the highest.
- Use the mnemonic 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, etc. to remember the order of filling.
- Remember the exceptions: Half-filled or full-filled orbitals have extra stability, leading to deviations like Cr and Cu.
💡 Note: Be aware of the Hund’s rule when filling degenerate orbitals; electrons will singly occupy orbitals of equal energy before pairing up.
2. Memorize the Periodic Table

A well-versed understanding of the periodic table can make the process of determining electron configurations much simpler:
- Each group (column) in the periodic table corresponds to the filling of the outermost shell or subshell.
- S-block elements fill the s orbitals, p-block elements fill p orbitals, and so on for d-block (transition metals) and f-block (inner transition metals).
| Element Type | Subshell Being Filled |
|---|---|
| Main Group (s & p block) | s and p orbitals |
| Transition Metals (d block) | d orbitals |
| Inner Transition Metals (f block) | f orbitals |

3. Utilize Electron Dot Diagrams

These diagrams, also known as Lewis dot structures, can help visualize the valence electrons:
- Valence electrons are the outermost electrons involved in bonding.
- Place the symbol of the element in the center and dots around it to represent the valence electrons.
- This method is particularly useful when dealing with atoms in groups 1 and 2, and groups 13 to 18.
📘 Note: For main group elements, the number of valence electrons is equal to the element’s group number.
4. Practice with Periodic Table Abbreviations
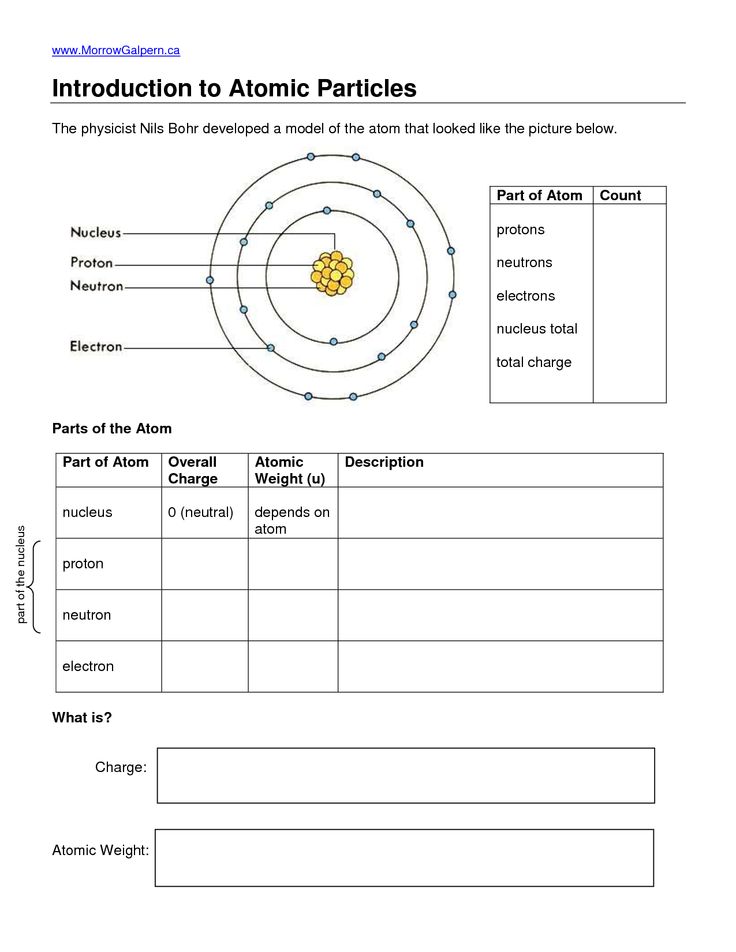
The periodic table abbreviations, like [Ne] for neon, can simplify writing electron configurations:
- Use these shorthand notations to represent the electron configuration of the noble gas preceding the element of interest.
- For example, sodium would be written as [Ne] 3s1.
👉 Note: Ensure to understand the electron configurations of the noble gases to correctly apply this method.
5. Incorporate Electron Configuration and Energy Level Diagrams

Visual representation can help in understanding the spatial arrangement of electrons:
- Draw energy level diagrams to show the distribution of electrons in different orbitals.
- Use arrows for electron spins in the diagrams to comply with the Pauli Exclusion Principle.
Here’s a simple energy level diagram for lithium:

🔍 Note: Energy level diagrams are crucial for understanding complex electron configurations in transition metals.
The journey through electrons in atoms worksheets teaches not just the mechanics of atomic structure but also enriches your grasp on the periodic table and its predictive power. Each tip provided here aims to make this journey smoother and more insightful, turning potentially tedious tasks into opportunities for learning. By applying the Aufbau principle, memorizing the periodic table, using electron dot diagrams, shorthand notations, and energy level diagrams, you equip yourself with tools that will serve you well beyond worksheet exercises. These practices will enhance your ability to understand and apply concepts of atomic theory, making you more proficient in chemistry and prepared for complex problems.
Why is understanding electron configuration important?

+
Electron configurations dictate the chemical behavior of atoms, including reactivity, bond formation, and the type of compounds an element can form. Understanding them helps in predicting chemical properties and reactions.
What is Hund’s rule?

+
Hund’s rule states that electrons will singly occupy orbitals of equal energy before pairing up, to maximize the total spin. This rule applies to degenerate orbitals (e.g., p, d, and f orbitals).
Can an atom have more electrons than its atomic number?
+
Yes, an atom can gain extra electrons to form negative ions (anions). However, in its neutral state, the number of electrons equals the atomic number.

