Mastering Electronic Configurations with Our Interactive Worksheet

Delving into the realm of chemistry often brings us to the concept of electronic configurations, an essential part of understanding atomic structure. These configurations are not just random letters and numbers; they are a systematic way to depict how electrons are distributed in different energy levels around an atom's nucleus. Whether you're a student learning the basics or a professional chemist working with complex molecular structures, understanding electronic configurations is key.
The Basics of Electronic Configurations


Electronic configuration refers to the distribution of electrons of an atom or molecule in its atomic or molecular orbitals. Here’s a quick rundown on how it works:
- Shells: Electrons are arranged in energy levels or shells denoted by principal quantum numbers (n=1, 2, 3, etc.).
- Subshells: Within each shell, there are subshells labeled s, p, d, and f.
- Orbitals: Each subshell has a specific number of orbitals, which can hold up to two electrons each.
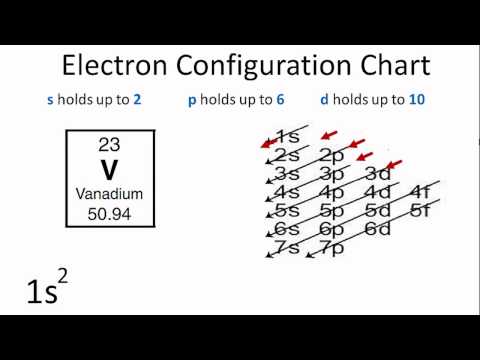
Understanding the Aufbau Principle

The Aufbau principle is like the foundational law for building an atom’s electronic configuration:
- Lower Energy First: Electrons fill lower energy orbitals before moving to higher energy ones.
- Order of Filling: The order generally follows the trend 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, etc.
💡 Note: While this order covers many elements, there are exceptions due to electron-electron interactions, especially in transition metals and lanthanides/actinides.
Hund’s Rule and the Pauli Exclusion Principle
Beyond the Aufbau principle, two other rules dictate the behavior of electrons in atoms:
- Hund’s Rule: Electrons in the same subshell will occupy all available orbitals singly before pairing up, and all electrons in singly occupied orbitals will have the same spin.
- Pauli Exclusion Principle: No two electrons in the same atom can have the same four quantum numbers. This means each electron in an orbital must have opposite spins.
Interactive Worksheet: A Fun Way to Learn


We’ve developed an interactive worksheet that allows you to:
- Configure electrons for elements up to atomic number 118.
- Explore exceptions in configurations for transition metals and inner transition elements.
- Test your knowledge with fill-in-the-blank, true/false, and short answer questions.
Practical Applications

| Application | Explanation |
|---|---|
| Chemical Bonding | Knowing electronic configurations helps predict the type of bonds atoms can form. |
| Spectroscopy | The emission and absorption of light by atoms are dictated by electron transitions between energy levels. |
| Periodic Table | It explains the periodic trends in reactivity and physical properties. |

📝 Note: Understanding electronic configurations also aids in the explanation of why some elements behave differently under similar conditions.
Summary of Key Points

In the journey through electronic configurations, we’ve covered:
- The principles guiding electron placement in atoms.
- The interactive worksheet as a tool for learning and testing knowledge.
- Real-world applications of electronic configurations in science and technology.
By grasping these concepts, you're not only enhancing your understanding of atomic structure but also equipping yourself with the knowledge to delve deeper into chemistry's intricacies. Electronic configurations aren't just academic; they are the backbone of understanding how matter interacts at the most fundamental level.
What is an electronic configuration?

+
An electronic configuration is the arrangement of electrons in energy levels, subshells, and orbitals around an atom’s nucleus, following specific rules like the Aufbau principle, Hund’s rule, and the Pauli exclusion principle.
Why do we need to know electronic configurations?

+
Understanding electronic configurations is crucial for predicting chemical reactivity, understanding periodic trends, and explaining various chemical phenomena like bonding and spectroscopy.
Can electronic configurations be predicted using the Periodic Table?

+
Yes, the Periodic Table provides a layout that largely follows the order of electron filling. However, exceptions exist due to electron-electron interactions, especially in transition metals.
How can interactive worksheets help in learning electronic configurations?

+
Interactive worksheets provide hands-on practice, immediate feedback, and a visual representation of electronic configurations, making the learning process engaging and effective.