5 Easy Tips for Mastering Electron Configuration Answers

Mastering electron configuration is a fundamental skill for anyone studying chemistry, physics, or related fields. It can seem daunting at first, but with the right approach, understanding how electrons are distributed in an atom's shells, subshells, and orbitals becomes second nature. Here, we'll explore five easy tips that can help you conquer the challenge of electron configurations, providing you with a structured path towards mastering this essential concept.
Understanding the Basics of Electron Configuration

Before diving into the tips, let's briefly review what electron configuration entails:
- Atomic Number: This indicates the number of protons and is crucial as it also determines the number of electrons in a neutral atom.
- Shells, Subshells, and Orbitals: Electrons fill into energy levels (shells), which are divided into subshells (s, p, d, f) with specific orbitals.
- Aufbau Principle: Electrons occupy the lowest energy orbitals first.
- Pauli Exclusion Principle: Only two electrons can occupy the same orbital, and they must have opposite spins.
- Hund's Rule: Electrons within the same subshell prefer to have their spins unpaired if possible, to minimize electron-electron repulsion.

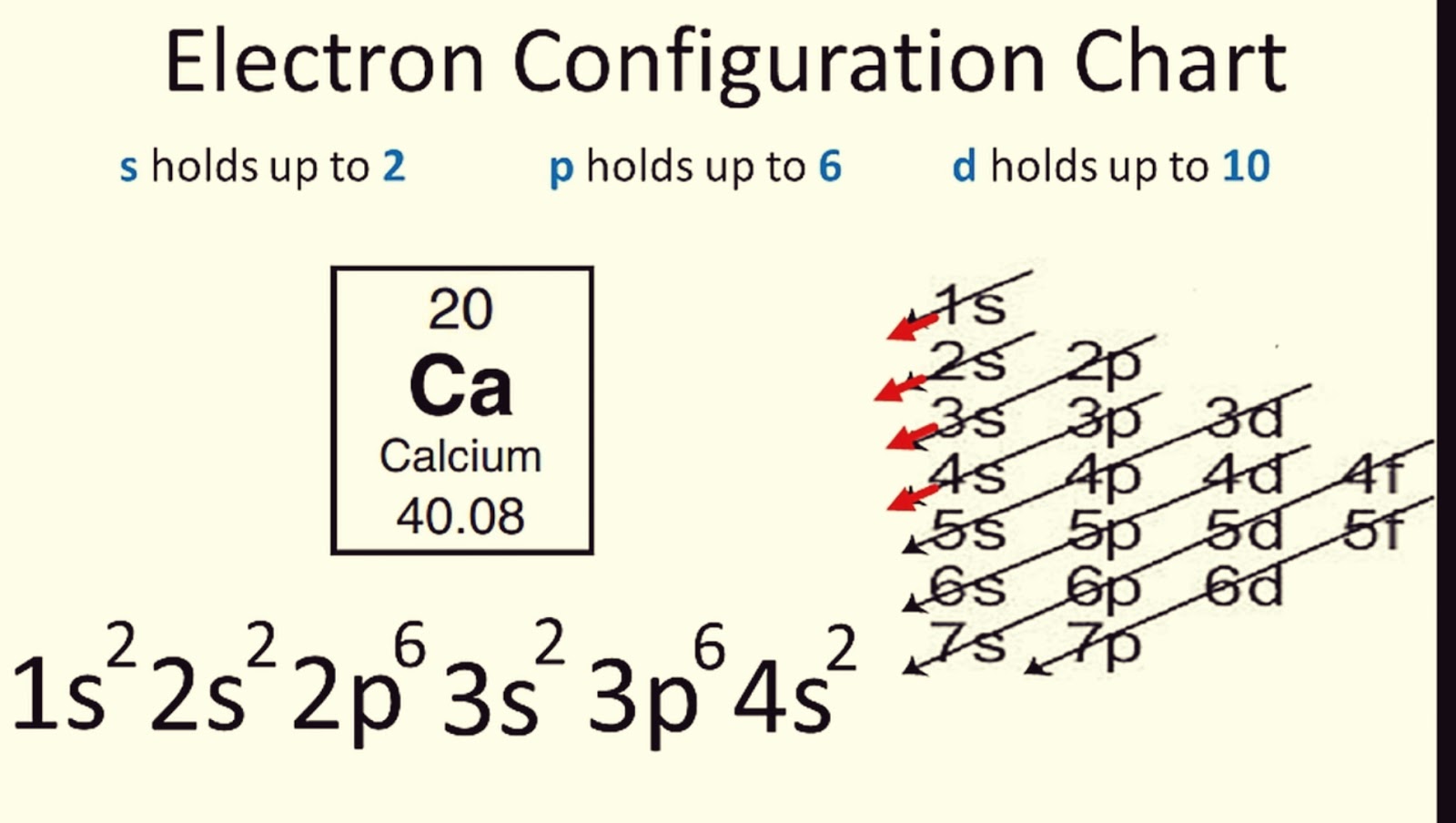
Tip #1: Utilize the Periodic Table

The Periodic Table is not just a tool for finding atomic weights or elements; it's your roadmap for electron configurations. Here’s how to use it:
- Groups: Elements in the same group often share electron configuration patterns, particularly in their outer shell.
- Blocks: The Periodic Table is divided into blocks (s, p, d, f) which correspond to the filling of subshells.
- Periods: Each row (period) corresponds to the principal energy level (shell number) of the atom.
⚗️ Note: For transition and inner transition metals, note the anomalies where they don't follow the expected order due to the energy levels overlap.
Tip #2: Master the Electron Configuration Notation

Knowing the notation for electron configurations will save time and help in visualizing the electron distribution:
- Noble Gas Shortcut: Use noble gas electron configuration as a base, then add electrons up to the element in question.
- Abbreviated Notation: e.g., for Sodium [Na]: 1s2 2s2 2p6 3s1 or using Neon as a base, [Ne] 3s1.
- Orbital Diagrams: Visualize electron distribution using boxes for orbitals and arrows for electron spins.

Tip #3: Memorize Key Elements and Trends

By memorizing a few key elements and trends, you'll enhance your ability to quickly determine configurations:
- Noble Gases: Understand their complete shells to use as shortcuts.
- Transition Elements: Recognize their irregularities.
- Atomic Trends: Learn how electron configuration impacts atomic properties like size, ionization energy, and electronegativity.
Tip #4: Practice Writing Electron Configurations

Regular practice is the key to mastery:
- Varied Examples: Write configurations for elements from different groups and periods.
- Element Identification: Given an electron configuration, identify the element.
- Interactive Tools: Use online tools or electron configuration calculators to verify your work.
Tip #5: Apply Concepts to Real-World Chemistry

Seeing how electron configurations apply to chemical reactions and properties:
- Chemical Bonding: Electron configurations explain why elements bond the way they do.
- Periodic Trends: Trends in electron configurations explain trends in reactivity, color, etc.
- Catalytic Activity: Understand how electron configurations affect the function of catalysts.
By mastering electron configuration through these tips, you not only prepare yourself for exams and assignments but also develop a deeper understanding of chemical principles. Electron configuration forms the basis for understanding reactivity, trends, and much of the chemistry that surrounds us. With consistent practice and application, the abstract nature of electron configurations will become a concrete tool in your chemical toolbox.
Keep in mind that mastering electron configuration isn't just about rote memorization. It's about recognizing patterns, understanding the exceptions, and applying these principles to broader chemical contexts. Embrace the journey of learning, and soon, electron configuration will no longer be a challenge but an insightful and fascinating part of your chemical knowledge.
Why do electron configurations help in understanding chemical behavior?

+
Electron configurations tell us how electrons are distributed within an atom, which in turn determines its chemical behavior. Elements with similar outer shell configurations tend to react similarly, explaining periodic trends and reactivity patterns.
How does the Periodic Table help in electron configuration?

+
The Periodic Table is organized by electron shell and subshell filling, which provides a visual representation of how electrons are added to elements. The position of an element can predict its electron configuration without having to remember each one individually.
What is the significance of Hund’s Rule?
+
Hund’s Rule states that electrons prefer to occupy orbitals singly before pairing up, which minimizes electron-electron repulsion and stabilizes the atom’s energy. This principle explains the magnetic behavior of atoms and the atomic structure in general.