5 Key Answers for Electron Configuration Orbital Diagram Worksheets

Electron configuration and orbital diagrams are fundamental concepts in chemistry that provide insights into the arrangement of electrons within atoms. Understanding these topics is crucial not only for academic purposes but also for applications in chemistry and physics, such as predicting the chemical behavior of elements or understanding electronic structures. This guide will cover the essential aspects of electron configurations and provide solutions to typical electron configuration orbital diagram worksheets to help clarify these concepts.
Understanding Electron Configurations


An electron configuration denotes how electrons are distributed among an atom’s various atomic orbitals. Here’s how to interpret electron configurations:
- Orbital Notation: Each atomic orbital is represented by a letter (s, p, d, f) followed by a superscript indicating the number of electrons in that orbital.
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy level. This principle guides the sequential filling of electron shells.
- Hund’s Rule: When electrons occupy orbitals of equal energy, they fill them singly first before pairing up.
- Pauli Exclusion Principle: No two electrons can have the same set of four quantum numbers; hence, each orbital can contain a maximum of two electrons with opposite spins.
Steps for Filling Electron Configurations

Here’s a step-by-step guide to constructing electron configurations:
- Identify the atomic number: This tells you the number of electrons.
- Follow the Aufbau sequence: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f…
- Apply Hund’s Rule: Fill orbitals singly before pairing electrons.
- Respect the Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons with opposite spins.
Orbital Diagram Worksheets: Examples and Solutions

Let’s apply these principles to real-world examples using orbital diagrams:
1. Hydrogen (Atomic Number 1)

- Electron Configuration: 1s1
- Orbital Diagram:

2. Helium (Atomic Number 2)

- Electron Configuration: 1s2
- Orbital Diagram:

3. Oxygen (Atomic Number 8)
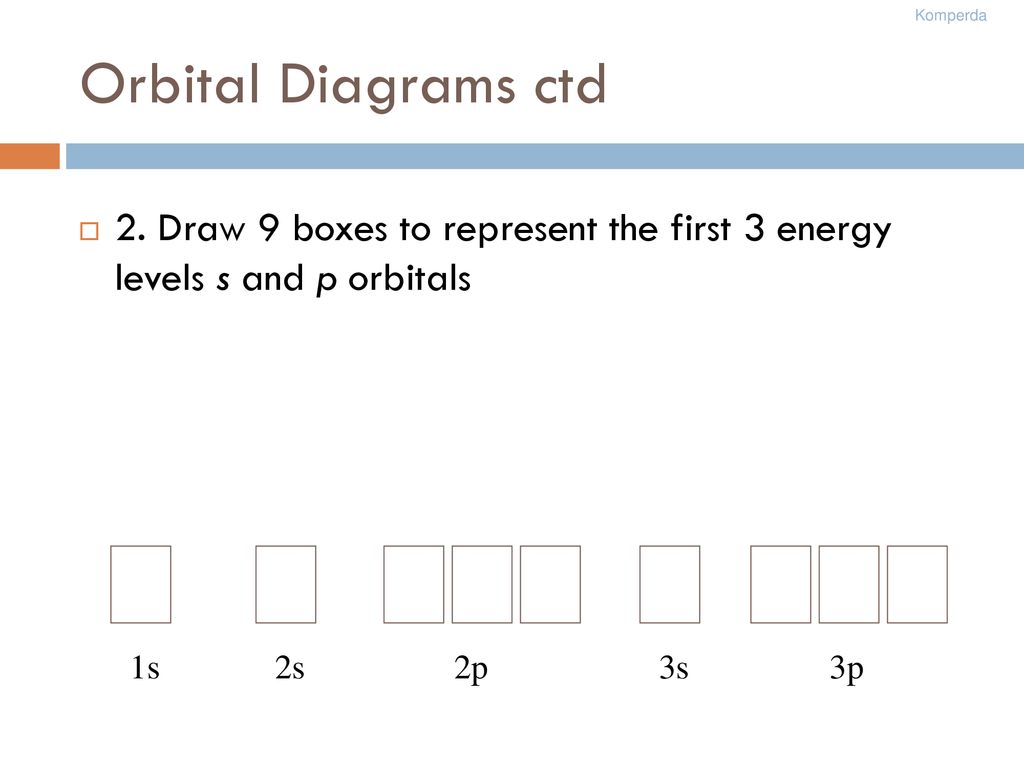
- Electron Configuration: 1s2 2s2 2p4
- Orbital Diagram:

4. Chromium (Atomic Number 24)

- Electron Configuration: 1s2 2s2 2p6 3s2 3p6 3d5 4s1
- Orbital Diagram:

🎯 Note: Chromium demonstrates an exception where the 4s orbital loses an electron to half-fill the 3d subshell, which provides additional stability to the atom.
5. Copper (Atomic Number 29)

- Electron Configuration: 1s2 2s2 2p6 3s2 3p6 3d10 4s1
- Orbital Diagram:

🎯 Note: Similar to chromium, copper undergoes an exception where an electron from the 4s moves to the 3d to achieve full or half-filled subshells for increased stability.
To summarize, electron configuration provides a detailed map of where electrons reside in an atom. Learning how to construct and interpret these configurations is key to mastering chemistry, particularly for understanding atomic structure, chemical bonding, and the periodic table. Through the examples and principles outlined, you're now better equipped to tackle electron configuration orbital diagram worksheets, understanding both the standard rules and the exceptions that make chemistry a dynamic field.
What does the notation [He] 2s2 2p4 mean in electron configurations?

+
The notation indicates that the atom has the same electron configuration as helium (1s2) and then two more electrons in the 2s orbital and four in the 2p orbital.
Why are there exceptions to the typical electron filling patterns in some atoms like Chromium or Copper?

+
These exceptions occur because having half-filled or completely filled subshells provides extra stability to the atom due to electron-electron interactions and the symmetrical distribution of electrons.
How does the electron configuration relate to an element’s position in the periodic table?

+
An element’s position in the periodic table corresponds to the energy level and type of the outermost (valence) electrons, directly related to the electron configuration.
Is it important to know exceptions in electron configurations for exams?

+
Yes, understanding exceptions can be crucial for higher-level chemistry courses, as they represent real-world atomic behavior that impacts the properties and reactivity of elements.
Can electron configuration predict chemical properties?

+
Absolutely, electron configuration provides insights into the valence electrons, which dictate how an element will form bonds, its reactivity, and its chemical properties.