7 Tips for Mastering Electron Configurations
Electron configurations might sound like one of the more esoteric parts of chemistry, but they're foundational for understanding the periodic table, chemical reactions, and the properties of elements. Whether you're a student, a chemist, or just someone interested in the fascinating world of atoms, mastering electron configurations can deepen your comprehension of how matter behaves. Here are seven tips to help you excel in this area:
1. Understand the Basics of the Atomic Structure


Before diving into electron configurations, it’s crucial to understand what you’re dealing with:
- Nucleus: Comprises protons and neutrons, the heart of the atom.
- Electrons: These negatively charged particles whirl around the nucleus.
- Energy Levels (Shells): Electrons reside in different energy levels or “shells” labeled K, L, M, N…
🔬 Note: Electrons fill up these shells in a specific order based on their energy levels.
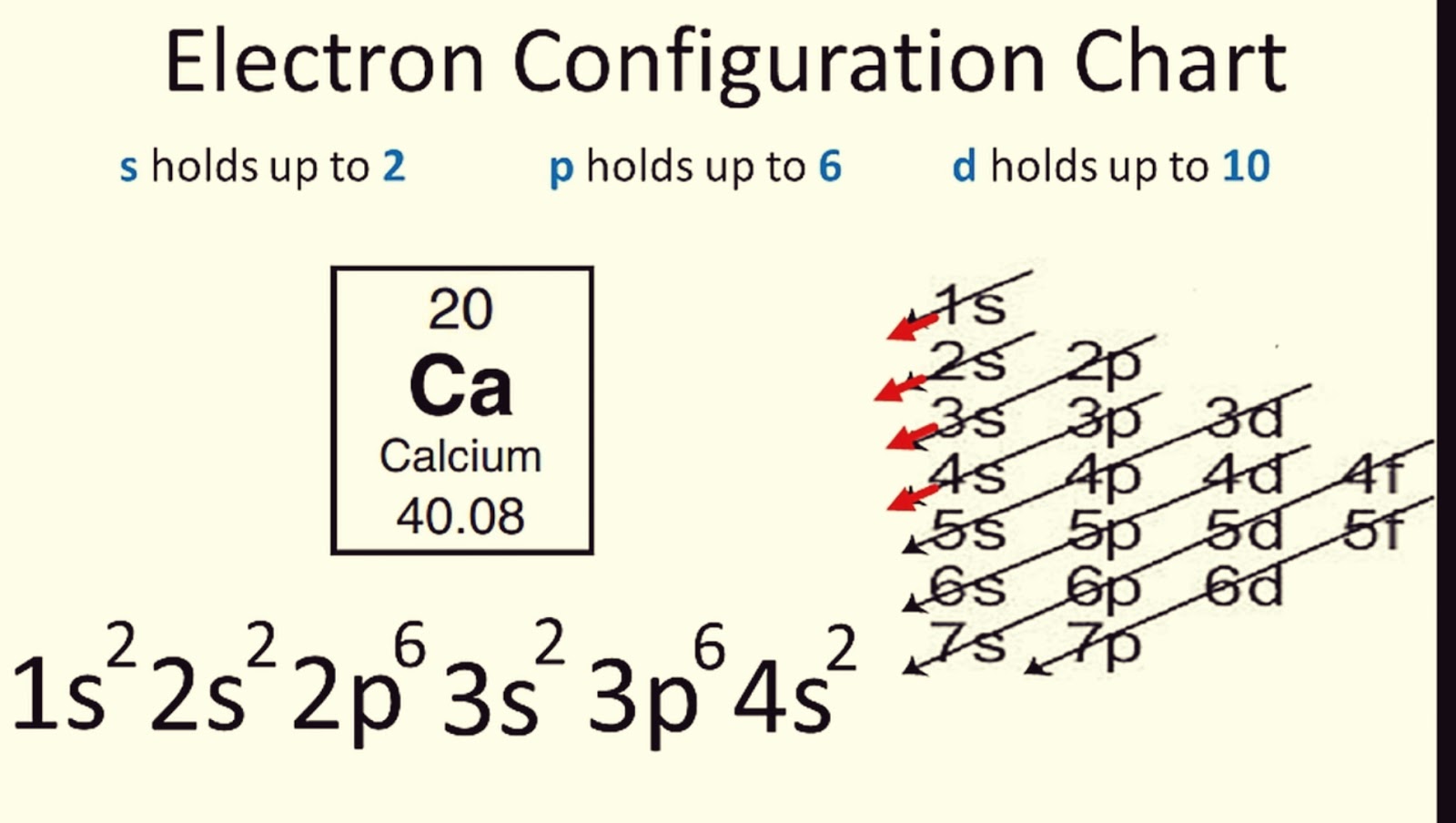
2. Learn the Aufbau Principle

The Aufbau Principle dictates how electrons fill up the atomic orbitals:
- Electrons fill the lowest energy orbitals first.
- The order follows this pattern: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, etc.
To help remember this, you might use the “Aufbau Diagonal Rule” or the “ladder” method where:
| Shell | Subshells (Orbitals) |
|---|---|
| n=1 | 1s |
| n=2 | 2s, 2p |
| n=3 | 3s, 3p, 3d |
| n=4 | 4s, 4p, 4d, 4f |
🔬 Note: The 3d orbital is filled after the 4s, even though it’s higher energy, due to this principle.
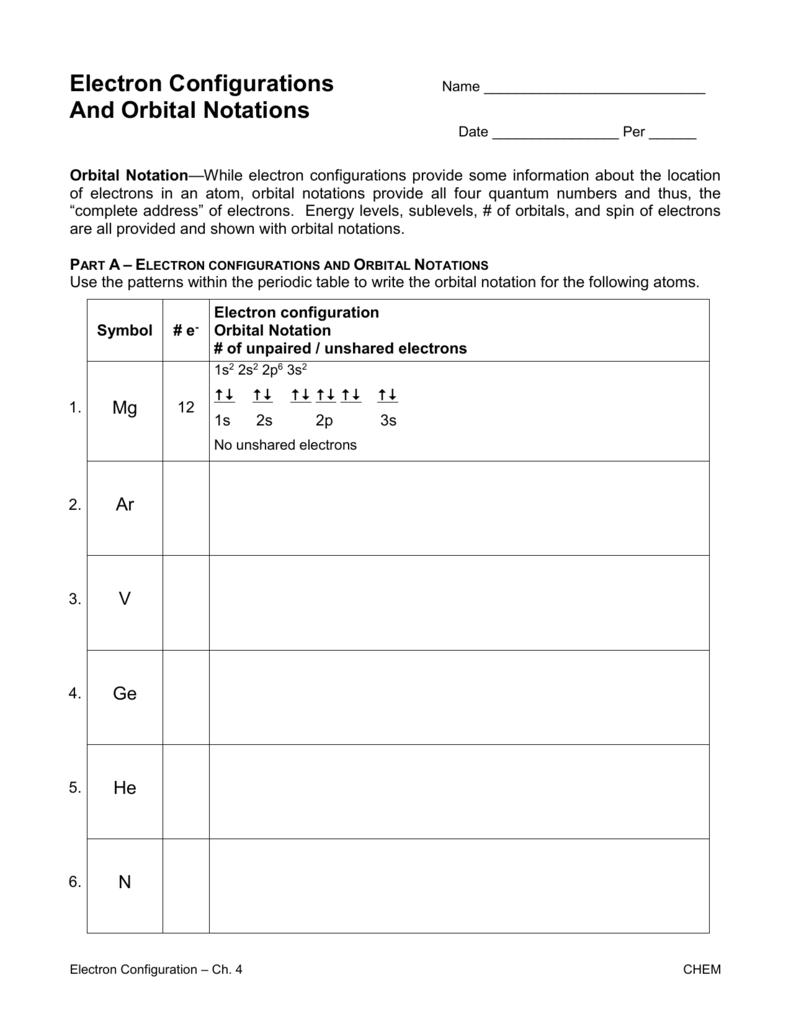
3. Master the Orbital Notation

Knowing how to write electron configurations using orbital notation is key:
- 1s²: This means there are 2 electrons in the 1s orbital.
- [He]2s²: The [He] represents the electron configuration of helium (1s²), followed by the 2s².
- [Kr]5s² 4d¹⁰ 5p⁶: Noble gas notation for elements beyond period 4.
Learning this notation helps visualize where electrons are in an atom.
4. Apply Hund’s Rule and the Pauli Exclusion Principle
- Hund’s Rule: Electrons will occupy separate orbitals within a subshell before pairing up.
- Pauli Exclusion Principle: No two electrons in an atom can have all four quantum numbers identical; hence, paired electrons must have opposite spins.
5. Use the Periodic Table

The periodic table can be an excellent tool:
- Each row (period) corresponds to the filling of another energy level.
- Blocks (s, p, d, f) indicate which subshells are being filled.
- Transition metals often have d-block electrons involved in their configuration.
6. Practice Writing Configurations

Practice really is key:
- Write configurations for elements from Hydrogen to Radon.
- Compare configurations to understand trends in electron filling.
- Look for exceptions, like Chromium (Cr) or Copper (Cu), where electrons “steal” into subshells to gain stability.
7. Understand Anomalies

Not every element follows the rules perfectly:
- Chromium and Copper: Due to the stability of half-filled or fully filled d-orbitals, they deviate from normal filling.
- Transition Elements: You’ll often find electrons in lower energy d-orbitals instead of following the Aufbau principle strictly.
🔬 Note: Anomalies can provide deep insights into electron stability and electron behavior.
By integrating these seven tips into your study of electron configurations, you'll gain a comprehensive understanding of how atoms are structured. This knowledge not only helps in mastering chemical concepts but also in appreciating the intricate nature of the world at the atomic level. Through practice, a keen eye for exceptions, and utilizing the periodic table as a map, electron configurations can become less of a puzzle and more of a fascinating narrative about the building blocks of our universe.
Why do we need to study electron configurations?

+
Understanding electron configurations is fundamental to predicting chemical behavior, reactivity, and understanding the periodic trends in the elements.
How can I remember the Aufbau filling order?

+
The “diagonal rule” or mnemonic device like “1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p…” helps in memorizing the order in which subshells are filled.
What are the most common exceptions to electron configuration rules?

+
Elements like Chromium (Cr) and Copper (Cu) show deviations due to the increased stability of half-filled or fully filled d-orbitals.