Mastering Electron Arrangement: Worksheet Guide

Mastering the concept of electron arrangement is crucial in understanding chemistry at an atomic level. Electrons, the negatively charged particles, orbit the nucleus of an atom in specific energy levels or shells. Knowing how electrons are arranged not only helps in predicting chemical behavior but also in understanding periodic trends. In this comprehensive guide, we'll delve into the intricacies of electron arrangement, providing practical worksheets and explanations to enhance your knowledge.
Electron Shells and Orbitals
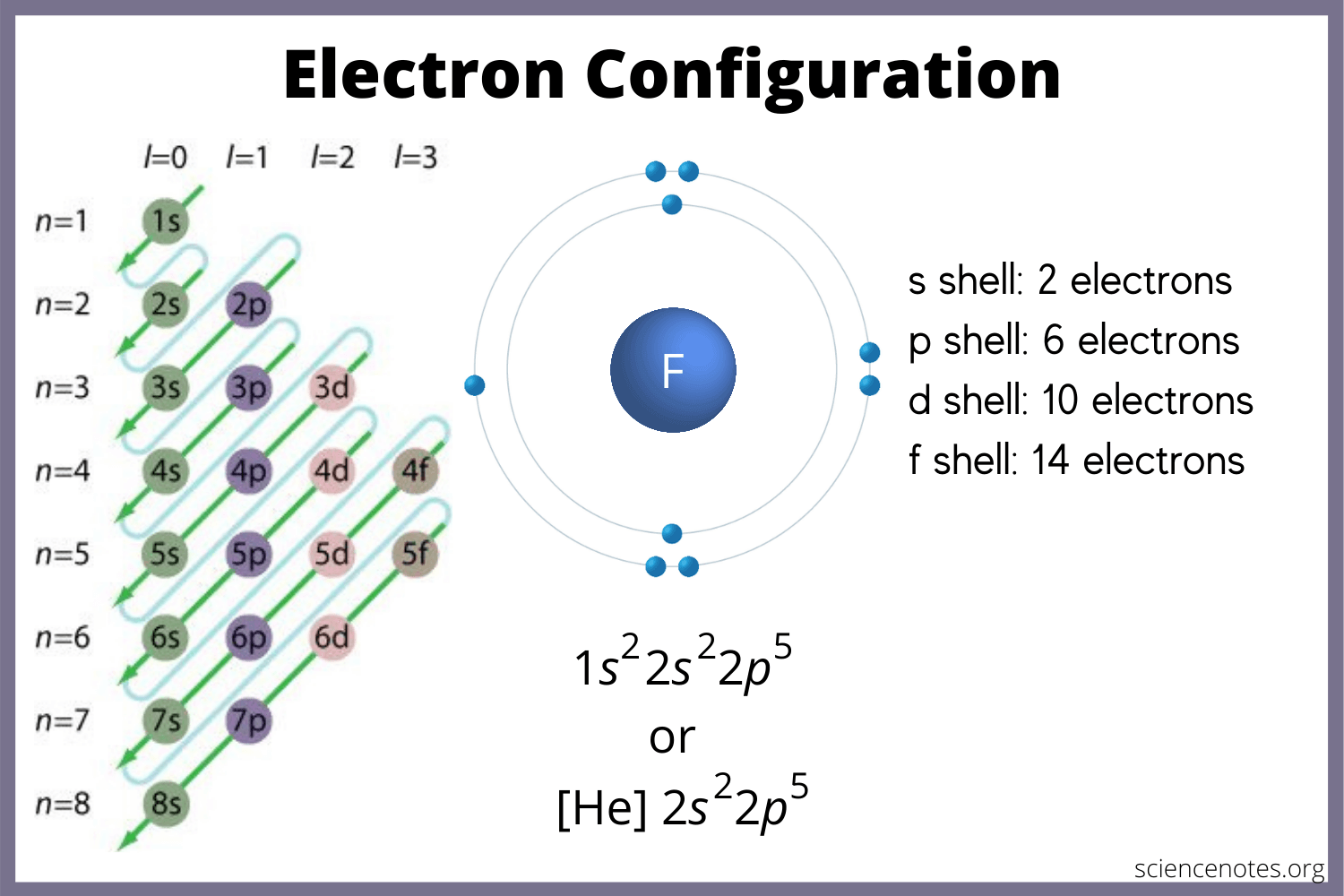

The concept of electron shells revolves around the idea that electrons exist in distinct layers around the nucleus. Here’s how they are structured:
- First Shell (K Shell): Holds up to 2 electrons.
- Second Shell (L Shell): Can accommodate up to 8 electrons.
- Third Shell (M Shell): Contains up to 18 electrons, but this number can decrease due to the stability rules (octet rule).
📘 Note: Beyond the third shell, things get a bit complex as electrons start filling the subshells, which include s, p, d, and f orbitals.
Orbital Notation

Each shell is composed of orbitals, which are specific paths that electrons follow within a shell:
- s orbitals: Can hold up to 2 electrons.
- p orbitals: Can hold up to 6 electrons (2 in each of the three p orbitals).
- d orbitals: Can hold up to 10 electrons.
- f orbitals: Can hold up to 14 electrons.
Electrons are assigned to orbitals following the Aufbau principle, Pauli exclusion principle, and Hund’s rule for maximum stability:
- Aufbau Principle: Electrons fill the lowest energy orbitals first.
- Pauli Exclusion Principle: Each orbital can contain a maximum of two electrons, with opposite spins.
- Hund’s Rule: For orbitals of equal energy, electrons will occupy each orbital singly before pairing up.
Writing Electron Configurations

To write electron configurations, you need to know:
- The order of filling energy levels.
- The number of electrons in each shell.
- The distribution of electrons among orbitals.
Let’s look at the example of writing the electron configuration for Sodium (Na):
| Shell | Subshells | Electron Configuration |
|---|---|---|
| 1 | 1s | 1s² |
| 2 | 2s, 2p | 2s² 2p⁶ |
| 3 | 3s | 3s¹ |

🔔 Note: Sodium (Na) has an atomic number of 11, which means it has 11 electrons. The configuration stops at 3s¹ because only one electron is needed to fill the 3s orbital.
Worksheet Practice

Here are some exercises to solidify your understanding of electron arrangement:
Exercise 1: Basic Electron Configuration

Write the electron configurations for the following elements:
- Helium (He)
- Carbon ©
- Potassium (K)
Answer Key:
Helium (He) - 1s²
Carbon © - 1s² 2s² 2p²
Potassium (K) - 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹
Exercise 2: Orbitals and Subshells

Identify the number of electrons in each subshell for the following elements:
- Chlorine (Cl)
- Argon (Ar)
Answer Key:
| Element | 1s | 2s | 2p | 3s | 3p | 4s | 3d |
|---|---|---|---|---|---|---|---|
| Chlorine (Cl) | 2 | 2 | 6 | 2 | 5 | ||
| Argon (Ar) | 2 | 2 | 6 | 2 | 6 |
🕵️♂️ Note: Notice that Chlorine has an unpaired electron in the 3p subshell, making it more reactive than Argon, which has a full outer shell.
Periodic Trends and Electron Configurations
Understanding how electrons are arranged within atoms helps in recognizing patterns in the periodic table:
- Atomic Radius: Increases down a group due to the addition of electron shells, but decreases across a period due to increasing effective nuclear charge.
- Ionization Energy: Generally increases across a period as the electrons are closer to the nucleus and thus harder to remove.
- Electronegativity: Also increases across a period but decreases down a group.
📈 Note: Electron configuration directly influences these trends by determining how tightly electrons are held by the nucleus.
Conclusion

Understanding electron arrangement is fundamental to grasping the basics of chemical behavior and properties. Through the study of shells, subshells, and orbitals, we can predict how elements interact, form bonds, and exhibit periodic trends. This knowledge forms the backbone of chemistry, making it an invaluable tool for students and professionals alike. By working through the exercises and examples provided, you’ll be well on your way to mastering this essential aspect of chemistry.
Why are electron configurations important?

+
Electron configurations provide insights into an element’s chemical behavior, its ability to form bonds, and its reactivity. They help predict how elements will interact with each other and with compounds, crucial for chemistry, materials science, and various other fields.
What is the Aufbau principle?

+
The Aufbau principle states that electrons fill atomic orbitals in order of increasing energy levels. The order is usually 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, etc., following the diagonal rule.
How do you determine the number of electrons in each shell?
+
Each shell can hold a specific number of electrons: 1st shell (K) - 2 electrons, 2nd shell (L) - 8 electrons, 3rd shell (M) - 18 electrons (though typically follows the octet rule). Beyond that, electrons are distributed among subshells according to their capacity.
What happens when an atom gains or loses electrons?

+
When an atom gains electrons, it forms a negatively charged ion (anion). If it loses electrons, it becomes positively charged (cation). The electron configuration changes, affecting the atom’s reactivity and chemical properties.
Can electrons exist in between shells?

+
Electrons primarily occupy shells or orbitals with specific energy levels. However, in quantum mechanics, there is a concept of electron tunneling where an electron might temporarily move between allowed energy levels, but this isn’t considered as occupying a space between shells.