Master Electrochemistry with Our Cells Worksheet Guide

The realm of electrochemistry holds secrets to understanding some of the most fundamental processes in our universe, from how our bodies generate energy to the functioning of batteries that power our devices. For students and enthusiasts alike, diving into electrochemistry can be both exhilarating and intimidating. This guide aims to demystify the complex world of electrochemistry using our specially curated cells worksheet, making learning not only simpler but also more engaging. Whether you're prepping for an exam, pursuing a deeper understanding, or just curious about how things work at a molecular level, let's embark on this electrifying journey together.
Understanding Electrochemical Cells

Before we delve into the specifics of our worksheet, it’s essential to grasp the basic principles of electrochemical cells:
- What is an Electrochemical Cell? - An electrochemical cell is a device that converts chemical energy into electrical energy or vice versa through redox (oxidation-reduction) reactions. There are primarily two types: Galvanic (Voltaic) Cells, which generate electrical energy, and Electrolytic Cells, which require an external source of electrical energy to drive a chemical reaction.
- The Components:
- Anode - The electrode where oxidation occurs (loss of electrons)
- Cathode - The electrode where reduction occurs (gain of electrons)
- Electrolyte - A medium that contains ions, enabling the flow of electric charge
- Salt Bridge - Allows the movement of ions to balance charge in galvanic cells
- Basic Cell Operation:
- In a galvanic cell, spontaneous redox reactions drive the cell to produce electricity.
- In an electrolytic cell, electricity is used to drive a non-spontaneous redox reaction.

Using the Cells Worksheet
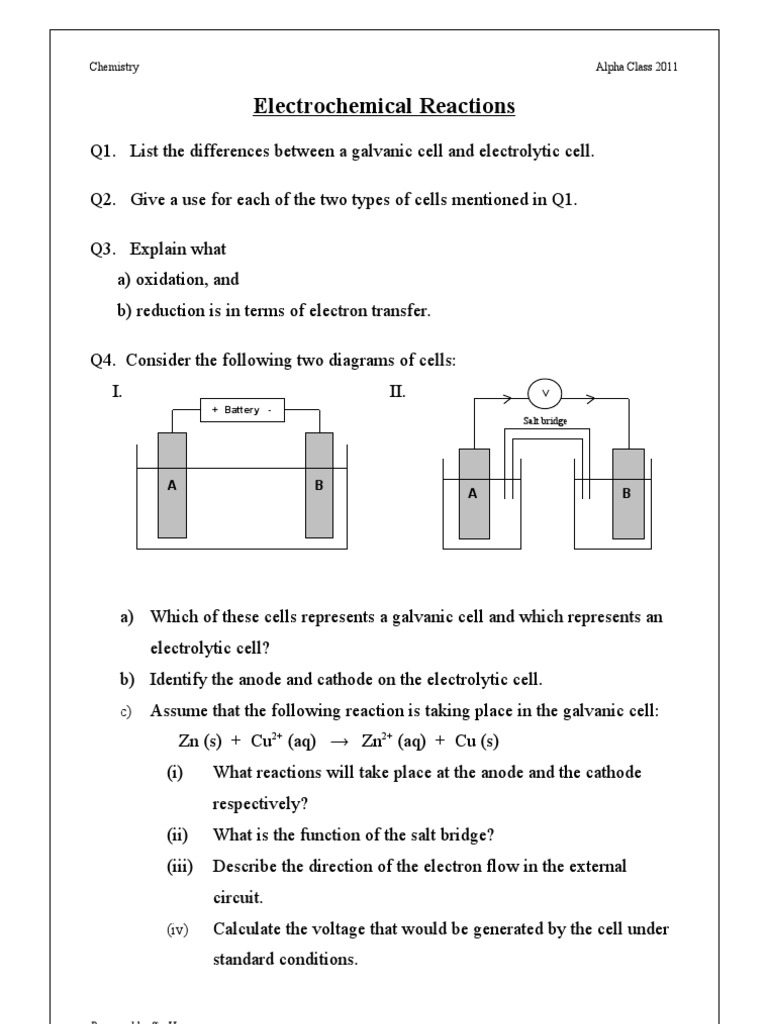
Now that you’re acquainted with the basics, let’s dive into how our cells worksheet can help you master electrochemistry:
- Problem Sets: The worksheet includes problems that vary from simple to complex, designed to reinforce your understanding of electrochemical cells through practical applications. Here’s how they’re structured:
- Identification of anodes and cathodes
- Balancing redox equations
- Calculating cell potential (E0cell) using the Nernst equation
- Diagrams: Each problem set is accompanied by diagrams that visualize the cell setup, aiding in visual learning.
- Data Tables: For reference, the worksheet includes a table of Standard Reduction Potentials which is crucial for solving cell potential problems. Here’s a simplified version:
| Half-Reaction | E0 (Volts) |
|---|---|
| F2 (g) + 2e- -> 2F- (aq) | +2.87 |
| O2 (g) + 4H+ (aq) + 4e- -> 2H2O (l) | +1.23 |
| Ag+ (aq) + e- -> Ag (s) | +0.80 |

- Explanation of Concepts: Besides problem-solving, the worksheet provides brief explanations of key electrochemistry concepts such as standard electrode potential, cell notation, and electrode reactions.
⚡ Note: When working through the problems, keep a reference of Standard Reduction Potentials handy. This will help you calculate cell potentials accurately.
Applications of Electrochemical Cells

Eelectrochemistry has practical implications beyond academic curiosity:
- Batteries: From the lithium-ion batteries in your smartphone to the lead-acid battery in your car, understanding electrochemistry is essential for their design and operation.
- Corrosion Protection: Electrochemical reactions are responsible for both corrosion and its prevention through cathodic protection.
- Electroplating: Industries use electroplating for metal coating, enhancing the durability, aesthetics, or conductivity of materials.
To further your learning, the worksheet includes real-world scenarios and challenges you to apply your knowledge to solve practical problems.
💡 Note: Electrochemistry doesn't just sit in textbooks; its principles are applied in numerous industries and everyday technologies, making it an exciting area of study.
Wrapping up, mastering electrochemistry through our cells worksheet guide offers a structured approach to learning about this fascinating subject. By understanding the basics of electrochemical cells, delving into problem sets, and exploring the practical applications, you can unlock the secrets behind batteries, fuel cells, corrosion, and more. Whether you're preparing for exams or simply enriching your knowledge, this guide aims to provide clarity, deepen understanding, and spark curiosity in the electrifying world of electrochemistry.
How do I identify the anode and cathode in an electrochemical cell?

+
In a galvanic cell, the anode is where oxidation (loss of electrons) occurs, usually marked as the negative electrode, while the cathode is where reduction (gain of electrons) happens, typically the positive electrode. Remember, ‘An Ox’ for anode oxidation and ‘Red Cat’ for reduction cathode.
What is the role of a salt bridge in an electrochemical cell?

+
A salt bridge maintains electrical neutrality by allowing ions to flow between the half-cells. It prevents the cell from building up excess charge and ensures that the redox reactions can continue to generate electricity.
How can I calculate the standard cell potential?

+
To calculate the standard cell potential (E0cell), you subtract the standard reduction potential of the anode from that of the cathode: E0cell = E0reduction(cathode) - E0reduction(anode).