Master Chemical Equation Balancing: Free Simple Worksheet

In the fascinating realm of chemistry, balancing chemical equations is a foundational skill that students must master. It's like fitting pieces of a puzzle together, ensuring that matter is conserved, and reactions remain in harmony with the laws of nature. This comprehensive guide delves into the essentials of balancing equations, enriched with a free worksheet to aid learning.
Understanding Chemical Equations


Before diving into balancing, let's clarify what a chemical equation represents. It's essentially a symbolic depiction of a chemical reaction where reactants on the left side turn into products on the right. Each substance is shown with its chemical formula, and the entire equation must follow the law of conservation of mass.
- Reactants: The initial substances that react with each other.
- Products: The resulting substances after the reaction takes place.
The Art of Balancing

Balancing a chemical equation means adjusting the coefficients (numbers preceding each molecule or atom) to ensure the number of atoms for each element is the same on both sides of the equation. Here's how you can tackle this:
- Count the atoms of each element on both sides of the equation.
- Change the coefficients to balance the equation, starting with the most complex molecule.
- Check and adjust until the number of each element is equal on both sides.
Steps for Balancing Equations


1. Count the Atoms
Identify how many atoms of each element are on the reactant and product side. This is the starting point for balancing.
2. Start with Complex Molecules
If there are polyatomic ions or complex molecules, balance them first, ensuring their counts are equal.
3. Balance Elements One by One
Systematically adjust coefficients to balance each element. Use fractions if necessary, then convert to whole numbers.
4. Verify and Double-Check
Once you believe the equation is balanced, recount the atoms to verify that they're indeed the same on both sides.
⚠️ Note: Never change the subscripts in a chemical formula as it alters the compound.
Simple Worksheet to Practice
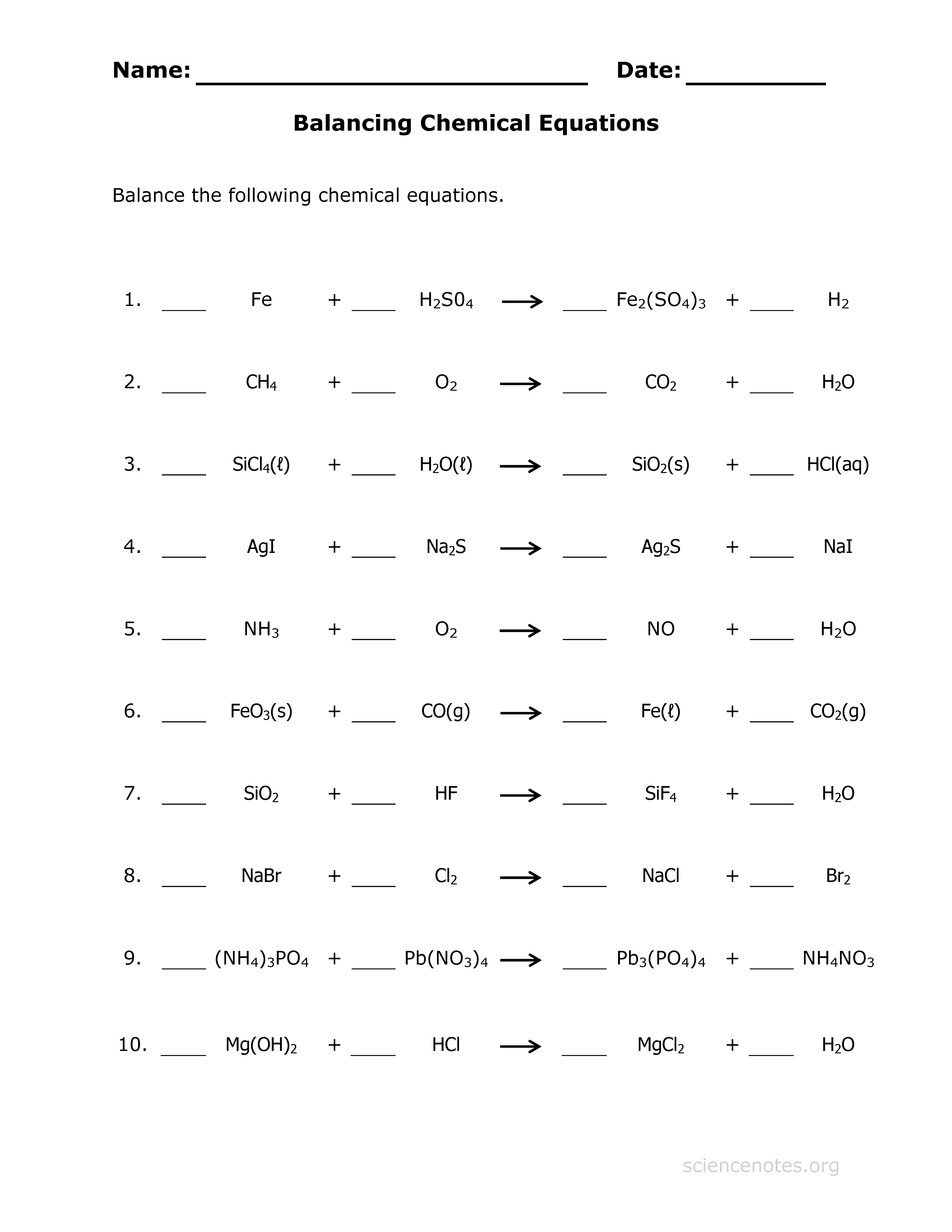
Mastering balance in chemical equations requires practice. Here's a free, downloadable worksheet crafted to help you hone your skills:
| Equation | Balanced Equation |
|---|---|
| H2 + O2 → H2O | 2H2 + O2 → 2H2O |
| Zn + HCl → ZnCl2 + H2 | Zn + 2HCl → ZnCl2 + H2 |
| Na + H2O → NaOH + H2 | 2Na + 2H2O → 2NaOH + H2 |

Strategies for Effective Balancing

- Methodical Approach: Balancing can be challenging, especially with multiple elements. Take your time and approach each element methodically.
- Check Even-Odd: If one side has an even number of atoms for an element and the other has an odd number, balance those atoms in pairs.
- Last Resort - Fractional Balancing: Use fractional coefficients as an intermediate step if the initial attempt doesn't balance with whole numbers.
🌟 Note: When fractions are used for balancing, multiply through the entire equation to convert to whole numbers.
Applications in Real Life

Balancing equations isn’t just an academic exercise; it has practical applications:
- Chemical Engineering: Designing reactors and processes relies on balanced chemical equations.
- Environmental Chemistry: Understanding pollutant transformations.
- Pharmaceuticals: Developing drugs where balancing reactions ensures the right chemical composition.
Throughout this journey of mastering chemical equation balancing, you've learned the core principles and practical methods for achieving equilibrium in chemical reactions. This knowledge forms the backbone of many chemistry-related disciplines and aids in a more profound understanding of how substances interact and transform. By consistently practicing with resources like the free worksheet, students can cement their grasp of these essential concepts.
Why do we need to balance chemical equations?

+
Balancing chemical equations ensures that the law of conservation of mass is adhered to. The reaction must conserve the same number of atoms of each element on both sides to represent the actual chemical process accurately.
Is there an easy method for balancing complex equations?

+
For complex equations, try balancing the elements that appear least frequently first, then work outward. If you’re stuck, a systematic trial-and-error approach or using software tools can help simplify the process.
Can we balance the equation by changing the subscripts?

+
No. Changing subscripts changes the identity of the compound, which is not what occurs during a chemical reaction. Only coefficients should be adjusted to balance equations.