7 Simple Tips for Drawing Covalent Bonds Correctly

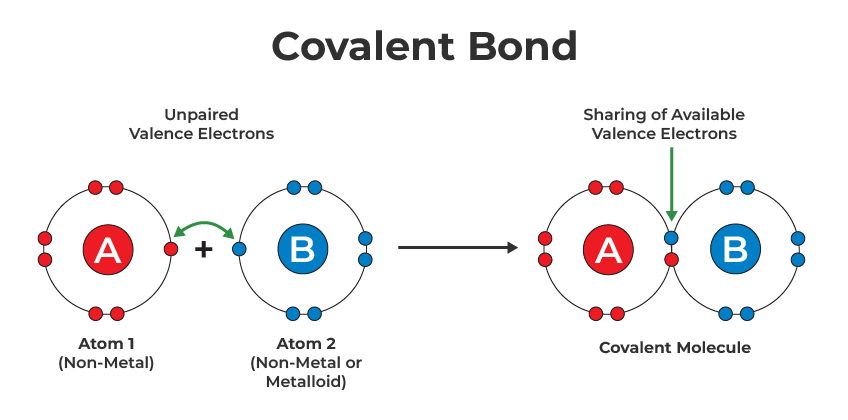
Learning to draw covalent bonds accurately is a crucial skill for students of chemistry. Covalent bonds, the sharing of electron pairs between atoms, form the backbone of molecular structures. Here's a step-by-step guide to help you master this fundamental chemical concept:
Understand the Basics


Before diving into drawing covalent bonds, ensure you have a firm grasp on:
- Atomic structure: Know the location of protons, neutrons, and electrons.
- Valence electrons: Recognize these as the outer shell electrons involved in bonding.
- Octet rule: Atoms generally aim for an octet (8 electrons) to achieve stability.
- Electronegativity: This determines how electrons will be shared in the bond.
Identify the Elements

First, identify the atoms you’re working with. Are they from the same element or different ones? Knowing this will:
- Help you predict bond polarity.
- Indicate if the bond might be polar or non-polar.
- Determine the electron sharing pattern.
Draw Lewis Structures

Using Lewis structures is a visual approach to represent covalent bonding:
- Count the total number of valence electrons.
- Write down the atomic symbols of each element.
- Place electrons around atoms to form octets. Begin with single bonds.
- If needed, add double or triple bonds to fulfill the octet rule.
| Element | Valence Electrons |
|---|---|
| Hydrogen | 1 |
| Carbon | 4 |
| Oxygen | 6 |

✏️ Note: Always start with a single bond for atoms needing more electrons to complete an octet.
Balance Electronegativity

Consider the electronegativity of atoms to determine how electrons are shared:
- If atoms have similar electronegativities, the bond is likely non-polar.
- If one atom has a significantly higher electronegativity, the bond is polar.
This influences the bond angle and the molecular geometry.
Check Your Structure
Ensure your covalent bond drawing adheres to:
- The octet rule for most elements (exceptions: hydrogen, boron, etc.).
- The total number of valence electrons used matches the count from step 2.
- The formal charges on atoms make sense; aim for a neutral overall charge.
Use Resonance Structures if Applicable

In some cases, molecules can be represented by multiple Lewis structures known as resonance structures:
- These structures involve the same arrangement of atoms but different placements of electrons.
- They collectively represent the true structure of the molecule.
✏️ Note: Resonance structures indicate delocalization of electrons within the molecule.
Practice with Common Molecules

Draw the covalent bonds for common molecules like:
- Water (H2O):

- Ammonia (NH3):

- Methane (CH4):

✏️ Note: Regular practice with these structures helps in understanding bond angles, molecular shapes, and polarity.
Mastering the art of drawing covalent bonds requires understanding the atomic structure, the octet rule, and how electrons are shared. By following these seven steps, you'll be able to construct correct Lewis structures, assess bond polarity, and apply resonance where necessary. Remember, practice is key. With each molecule you draw, your ability to visualize atomic interactions will grow, allowing you to better predict and understand molecular properties.
Why is it important to balance electronegativity when drawing covalent bonds?

+
Electronegativity determines how electrons are shared in a covalent bond. If the difference is high, electrons are pulled towards one atom, creating a polar bond. Understanding this helps in predicting the behavior of molecules in terms of polarity, reactivity, and interactions.
Can covalent bonds have resonance structures?

+
Yes, some molecules can have resonance structures where electrons are delocalized over the atoms. This means the molecule’s actual structure is an average of these resonance forms.
What if an atom doesn’t follow the octet rule?

+
Some atoms like hydrogen, boron, and certain transition metals can have incomplete or expanded octets. These exceptions must be noted when drawing their covalent bonds.


