5 Tips for Mastering Drawing Atoms Worksheet Answers

Understanding and drawing atoms is a fundamental skill in chemistry education, providing students with a visual representation of the basic building blocks of matter. Here are five comprehensive tips that can help you master the atoms worksheet answers, ensuring you grasp both the theoretical and practical aspects of atomic structure:
1. Understand the Basics of Atomic Structure

Before diving into drawing atoms, it’s imperative to fully comprehend what makes up an atom:
- Nucleus: Contains protons and neutrons, which are collectively known as nucleons.
- Electrons: Orbit the nucleus in energy levels or shells.
- Atomic Number: Determines the number of protons, which equals the number of electrons in a neutral atom.
- Mass Number: The sum of protons and neutrons.
🧪 Note: Remember that electrons are arranged in shells according to specific rules, with the first shell holding up to 2, the second shell up to 8, and so on.
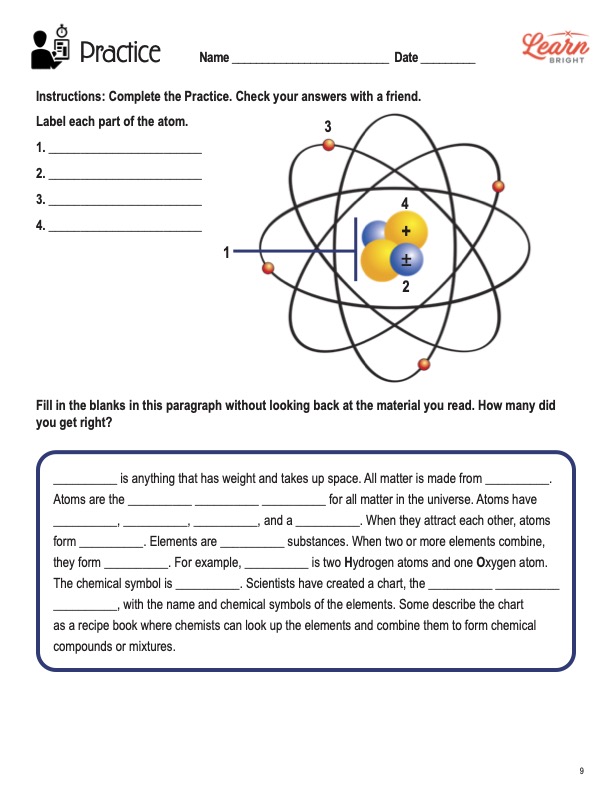
2. Practice Bohr’s Model of the Atom

One of the most straightforward models for beginners to start with is Bohr’s model:
- Draw the Nucleus: Place protons and neutrons in the center.
- Add Electron Shells: Draw concentric circles around the nucleus, each representing an energy level.
- Place Electrons: Allocate electrons to shells based on the atomic structure rules, starting from the innermost shell.
- Label Electrons: For accuracy, label each electron to keep track of its position.
⚗️ Note: Ensure you have the correct number of electrons in each shell to maintain atomic stability.
| Element | Protons | Neutrons | Electrons |
|---|---|---|---|
| Hydrogen (H) | 1 | 0 | 1 |
| Helium (He) | 2 | 2 | 2 |
| Lithium (Li) | 3 | 4 | 3 |

3. Master the Lewis Dot Structure

Lewis dot structures focus on the valence electrons:
- Determine Valence Electrons: Count the electrons in the outermost shell.
- Draw the Atom’s Symbol: Place the atom’s chemical symbol in the center.
- Place Dots: Use dots to represent each valence electron around the symbol.
- Check for Electron Pairs: Electrons pair up in the Lewis structure, so adjust your drawing accordingly.
🔬 Note: The number of dots around the symbol should match the number of valence electrons of the element.
4. Use Electron Configuration

This tip involves understanding how electrons fill up the energy levels:
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy level.
- Pauli Exclusion Principle: Each orbital can hold up to two electrons with opposite spins.
- Hund’s Rule: Electrons occupy unpaired orbitals of the same energy before pairing up.
- Noble Gas Notation: Simplifies the configuration by using the nearest noble gas as a shortcut.
Understanding these principles will help you to:
- Predict Electron Placement: Knowing how electrons are arranged can guide your drawings.
- Ensure Correct Shell Filling: The rules above help in accurately placing electrons in their respective shells.
📚 Note: Electron configurations can be complex for heavier elements, so practice with simpler atoms first.
5. Understand Isotopes and Ions

Lastly, remember that atoms can exist as isotopes (with different numbers of neutrons) or ions (with charges due to lost or gained electrons):
- Isotopes: Use the same Bohr or Lewis model but adjust the number of neutrons in the nucleus.
- Ions: Add or remove electrons from your drawings. Positive ions (cations) lose electrons, while negative ions (anions) gain electrons.
⚙️ Note: When drawing ions, pay attention to the charge to ensure the correct number of electrons are either added or subtracted.
In conclusion, mastering atoms worksheet answers involves a blend of theoretical understanding and practical application. By following these tips, you’ll gain a solid foundation in visualizing atomic structure, understand electron configurations, and accurately represent different forms of atoms like isotopes and ions. This knowledge not only helps in academic settings but also enhances your overall grasp of chemical principles.
How do I know which shell electrons go into?

+
Electron shell filling follows specific rules: the first shell can hold up to 2 electrons, the second up to 8, and the third up to 18. After that, each shell can hold up to 32 electrons. Electrons fill the lowest energy shells first.
What is the difference between an atom and an ion?
+
An atom is neutral, having an equal number of protons and electrons. An ion, on the other hand, has a charge because it has either lost or gained electrons, altering its electron-to-proton ratio.
Why are isotopes important?

+
Isotopes are crucial in many fields, including medicine (e.g., radioactive isotopes for imaging) and archaeology (e.g., radiocarbon dating). They allow us to trace the behavior of specific elements and study chemical reactions in detail.