5 Essential Tips for Mastering Diffusion Practice

Diffusion is a pivotal process in various scientific and practical fields, ranging from biological systems to industrial applications. Understanding how diffusion works can enhance our capabilities in research, industry, and daily life. Here are five essential tips to master the practice of diffusion, ensuring both precision and efficiency in your work.
1. Understanding Diffusion Basics
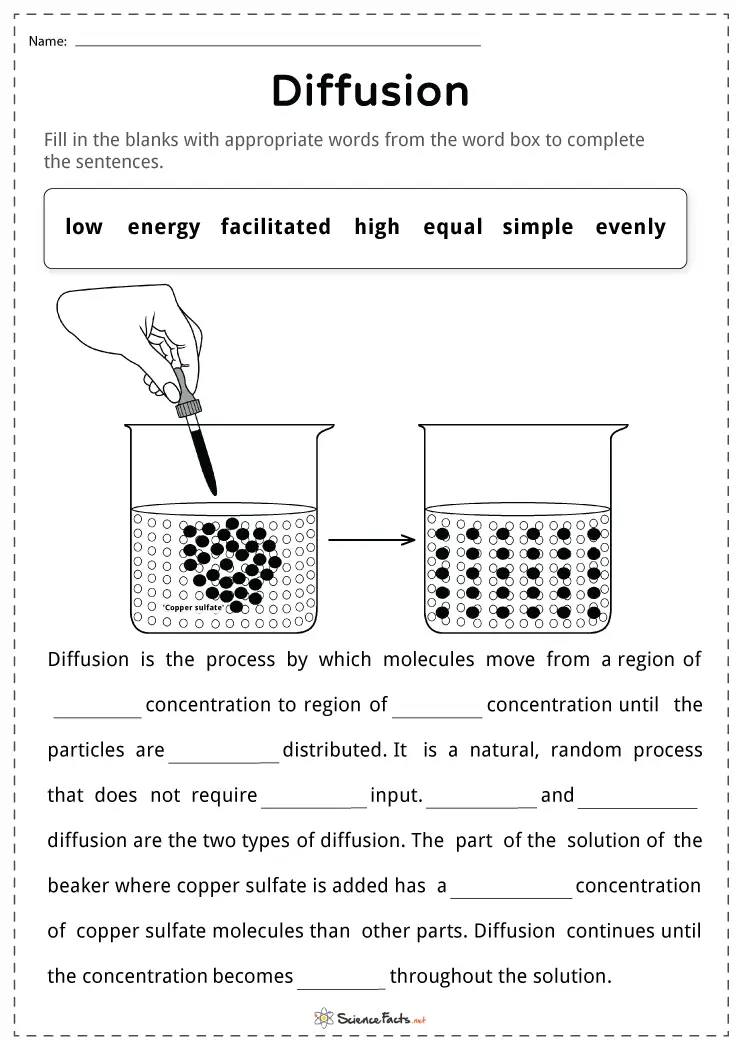
Before diving into the practical applications, grasping the basic principles of diffusion is crucial:
- Fick’s Laws: Fick’s first law relates the diffusive flux to the concentration gradient through the equation ( J = -D \frac{dc}{dx} ). Fick’s second law deals with the change in concentration over time due to diffusion.
- Types of Diffusion: There’s simple diffusion where molecules move randomly, facilitated diffusion with help from transport proteins, and active diffusion which involves energy to move molecules against a concentration gradient.
By understanding these fundamentals, you can better anticipate how substances will behave in different environments.
2. Choosing the Right Diffusion Model

Selecting an appropriate model for your specific application is key:
- Steady-State vs. Unsteady-State: Use steady-state models when the system remains in equilibrium or when you’re looking at long-term effects. Unsteady-state models are for analyzing how concentration changes over time.
- Geometric Considerations: The geometry of the system (e.g., planar, cylindrical, spherical) affects diffusion rates. Tailor your model to fit the physical setup of your experiment or system.
💡 Note: Understanding your system’s geometry can prevent errors in predictions about diffusion behavior.
3. Practical Diffusion Setup

Set up your diffusion experiment or process with these considerations:
- Control Environmental Variables: Temperature, pressure, and medium composition all impact diffusion rates. Ensure these variables are controlled or monitored.
- Material Selection: Select materials for your diffusion barrier or matrix. Permeability and porosity will influence diffusion rates significantly.
- Concentration Gradient Maintenance: Keep or establish a concentration gradient, as diffusion depends on it. This might involve constant replenishment of source material or removal of the diffusing substance.
4. Measurement Techniques

Measurement is crucial to quantify diffusion:
- Direct Methods: Techniques like UV-Vis spectroscopy or fluorescence microscopy directly measure concentration changes over time.
- Indirect Methods: Electrochemical methods or impedance spectroscopy can infer diffusion rates through related physical properties.
- Imaging: Techniques such as diffusion-weighted MRI or confocal microscopy provide spatial and temporal data on diffusion.
5. Data Analysis and Interpretation

Analyzing the results from your diffusion experiments requires:
- Fitting Data to Models: Use software or mathematical tools to fit experimental data to theoretical models for coefficient determination.
- Error Analysis: Quantify uncertainties and errors in your measurements to understand the reliability of your diffusion coefficients.
- Statistical Methods: Employ statistical techniques to validate your findings and understand the variability in your data.
📊 Note: Statistical analysis can significantly improve the credibility of your diffusion study results.
Mastering diffusion involves understanding its fundamentals, setting up controlled experiments, selecting the right measurement techniques, and meticulously analyzing data. Each step contributes to a comprehensive understanding and control over diffusion processes, whether you're investigating cellular mechanisms, optimizing industrial processes, or studying environmental impacts. Remember, diffusion is not just a phenomenon but a tool for exploring intricate systems and advancing various scientific disciplines.
What is the primary difference between simple and facilitated diffusion?

+
In simple diffusion, molecules move randomly due to their concentration gradient, requiring no energy. Facilitated diffusion, however, uses transport proteins to aid the movement, still following the concentration gradient but not requiring energy, unlike active transport which moves substances against the gradient.
Why is temperature important in diffusion processes?

+
Temperature increases molecular motion, thereby increasing the rate of diffusion. Warmer temperatures provide more kinetic energy to particles, speeding up the random movement of molecules which in turn promotes faster diffusion.
How can we measure diffusion in a non-invasive way?

+
Non-invasive techniques include diffusion-weighted MRI which uses the diffusion of water molecules in tissues to infer tissue properties, or fluorescence recovery after photobleaching (FRAP) in microscopy to study macromolecular mobility in cells.
What role does diffusion play in environmental systems?

+
In environmental systems, diffusion is critical for processes like nutrient distribution in soil and water bodies, pollutant dispersion, gas exchange in plant stomata, and the spread of contaminants in air or groundwater.
Can diffusion be manipulated to improve industrial processes?

+
Yes, diffusion can be controlled through temperature, concentration gradients, and the choice of materials to enhance efficiency in processes like drug delivery systems, waste treatment, and chemical reactions where even distribution of reactants is crucial.