5 Key Points for Diffusion and Osmosis Worksheet Answers
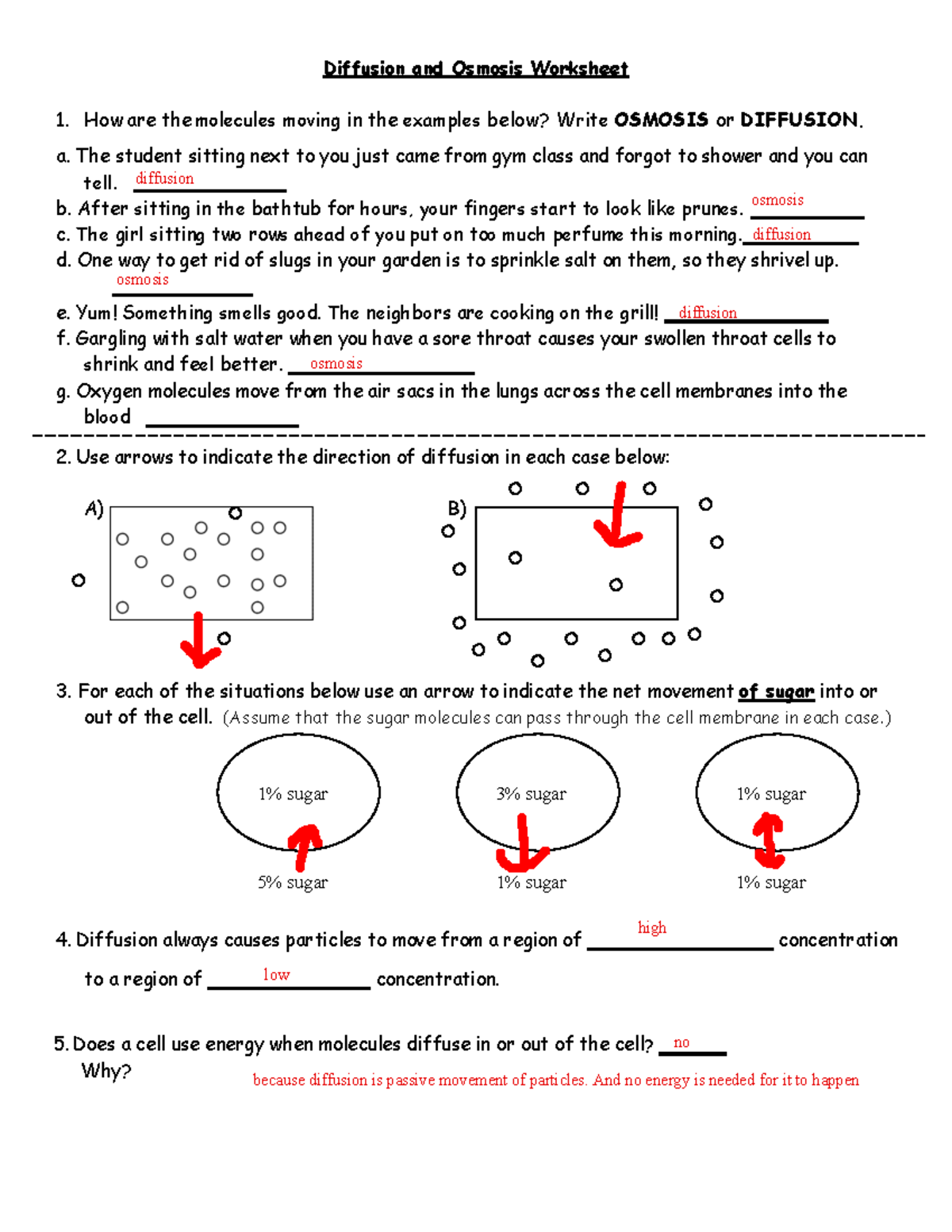
Understanding diffusion and osmosis is crucial for comprehending many biological processes, from the basic function of cells to complex systems like plant transport or animal kidney function. Both mechanisms involve the movement of substances through a semipermeable membrane, but they operate under different conditions. This long-form blog post will delve into the key points for answering questions typically found in a diffusion and osmosis worksheet, providing detailed explanations, practical examples, and relevant insights.
1. Defining Diffusion and Osmosis

Diffusion refers to the passive movement of particles from an area of higher concentration to an area of lower concentration until equilibrium is achieved. This principle applies not just to solutes but also to gases and heat, explaining many natural phenomena. For instance, when you spray perfume in one corner of a room, the scent eventually spreads evenly.
- The driving force for diffusion is the concentration gradient.
- It requires no energy; it's a passive transport mechanism.
- Molecules move in a random, but statistically significant manner.
Osmosis, on the other hand, is the movement of water through a semipermeable membrane due to a difference in solute concentration. Here, water molecules move from an area of lower solute concentration (hypotonic solution) to an area of higher solute concentration (hypertonic solution).
- The pressure that develops due to osmosis is called osmotic pressure.
- This process is also passive, needing no energy input.
- The semipermeable membrane allows water but restricts solute passage.
📚 Note: Although both diffusion and osmosis move substances, the primary difference is that osmosis deals specifically with the movement of water across a membrane.
2. Factors Affecting Diffusion Rate

To correctly answer questions regarding diffusion in worksheets, it’s vital to understand what impacts the rate at which substances diffuse:
- Concentration Gradient: The steeper the gradient, the faster diffusion will occur.
- Temperature: Higher temperatures increase kinetic energy, speeding up diffusion.
- Particle Size: Smaller particles diffuse more quickly than larger ones.
- Medium: The less viscous or more permeable the medium, the higher the diffusion rate.
- Distance: Shorter distances facilitate faster diffusion.
These factors are integral to predict how substances will behave in different scenarios, like in cell membranes or environmental systems.
3. Exploring Osmosis through Experiments

Osmosis can be illustrated effectively through experiments, which are often part of worksheets. One classic experiment involves:
| Setup | Observations | Conclusion |
|---|---|---|
| Placing an egg in distilled water and in concentrated salt solution separately | In distilled water, the egg swells; in salt solution, it shrivels | Water moves into or out of the egg based on solute concentration |
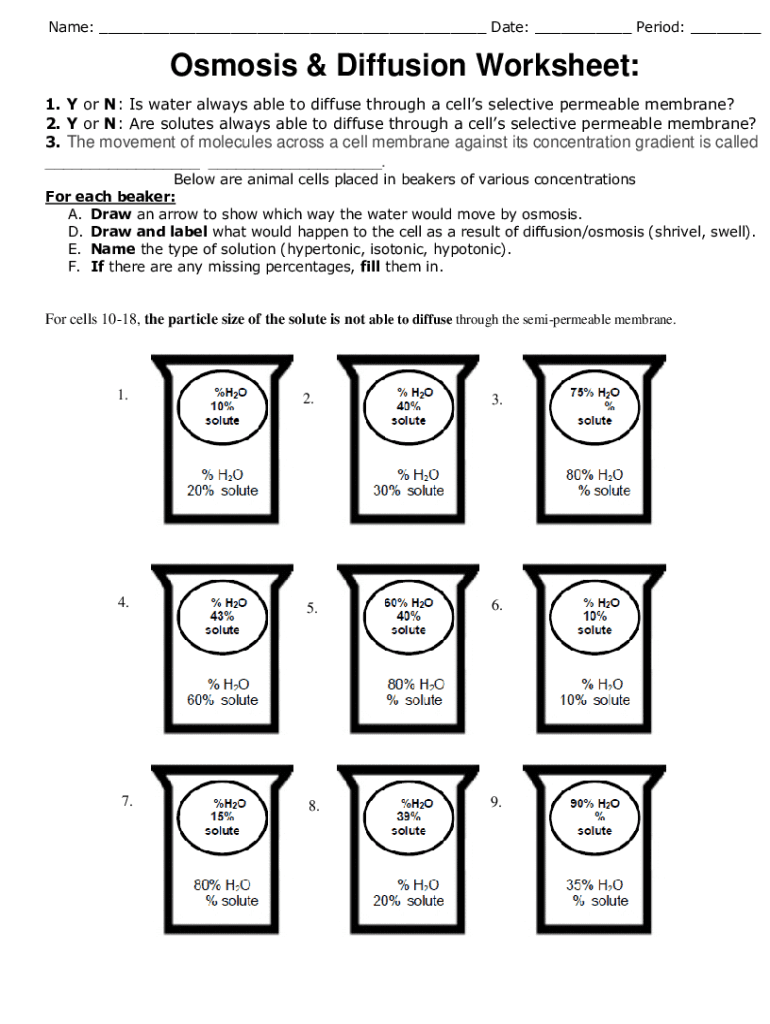
Such experiments help visualize osmosis's role in biological and ecological systems.
💡 Note: Always consider the nature of the membrane when dealing with osmosis. Not all membranes are equally permeable to solutes.
4. Applications in Biology

Diffusion and osmosis are not just academic topics; they are at the heart of several biological processes:
- Cellular Transport: Nutrients enter cells via diffusion, and waste products exit through this mechanism.
- Plant Function: Water uptake by plants occurs through osmosis, essential for plant growth and support.
- Animal Physiology: Kidneys utilize osmotic pressure to filter blood, reabsorb useful substances, and excrete waste.
Understanding these processes aids in interpreting biological phenomena and their impacts on health and ecology.
5. Real-World Examples and Anomalies

Beyond the lab and classroom, diffusion and osmosis play roles in real-life scenarios:
- Food Preservation: Salting or sugar curing prevents bacterial growth due to osmotic pressure.
- Desalination: Reverse osmosis is used to purify water by forcing it through a membrane to leave salts behind.
- Plants in Different Solutions: Turgor pressure in plants, influencing whether a plant stands upright or wilts, is an osmotic effect.
However, not all instances of water movement are purely osmotic, and some can be driven by other forces like capillary action or active transport.
As we wrap up this exploration into diffusion and osmosis, it's clear that these processes are fundamental to life. From the way our cells function to how we preserve food, manage ecosystems, and even in industrial applications, understanding diffusion and osmosis equips us with the knowledge to make better decisions, design efficient systems, and grasp the interconnectedness of biological phenomena. This knowledge is not just academic; it has practical implications in medicine, environmental science, and biotechnology.
What is the difference between diffusion and osmosis?

+
Diffusion is the passive movement of any particles from an area of higher concentration to an area of lower concentration, while osmosis specifically refers to the movement of water through a semipermeable membrane due to a difference in solute concentration.
Why does an egg swell in distilled water?

+
When placed in distilled water, an egg, which has a higher solute concentration inside, draws water in through osmosis, causing it to swell. This is because water moves from a region of lower solute concentration (distilled water) to a region of higher solute concentration (inside the egg).
How does osmosis play a role in kidney function?
+
The kidneys use osmosis to filter blood and concentrate urine. Water and small molecules pass through the semipermeable membrane of the glomerulus, but large molecules and blood cells remain in the blood. Later, through processes like countercurrent multiplication, water is reabsorbed back into the body, helping to maintain blood volume and concentration.