5 Ways to Master Diffusion and Osmosis Worksheet

Understanding the principles of diffusion and osmosis is crucial for students of biology, chemistry, and even those studying environmental science. These processes are fundamental to how substances move within and across cellular boundaries. Here's how you can master these concepts through worksheets designed to help you learn, practice, and test your understanding:
1. Utilize Interactive Simulations
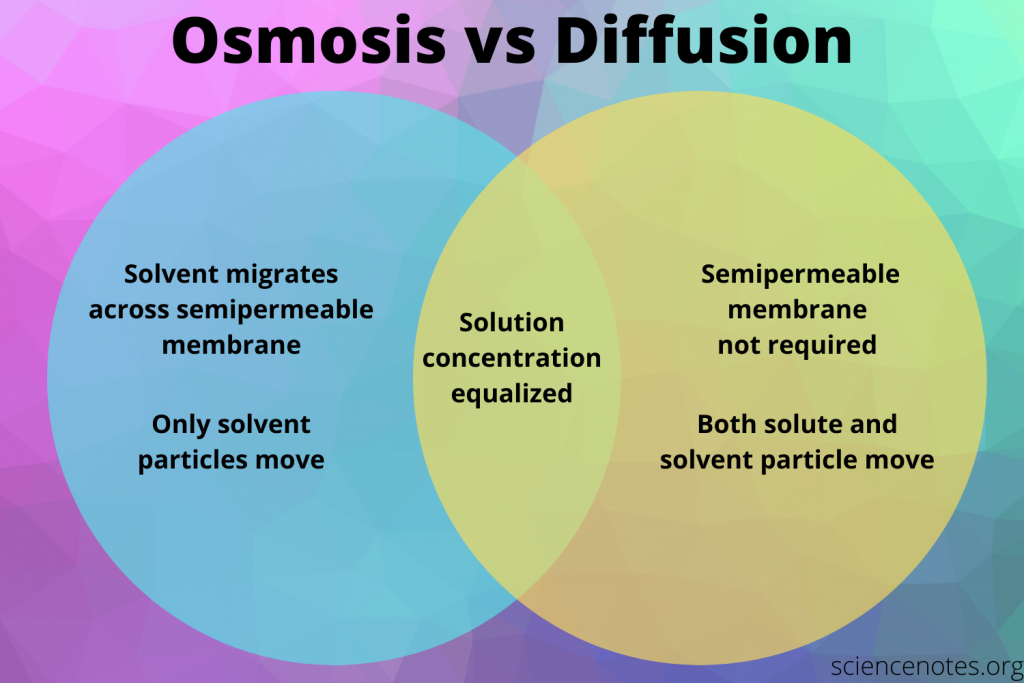

Before diving into worksheets, engage with online simulations to visualize diffusion and osmosis:
- Explore the Basics: Websites like PhET Interactive Simulations offer tools where you can manipulate variables to see how different conditions affect the rate and extent of diffusion and osmosis.
- Analyze Results: Pay attention to how concentration gradients, temperature, and membrane permeability alter the movement of particles.
- Take Notes: Document your observations to help with future worksheet exercises.
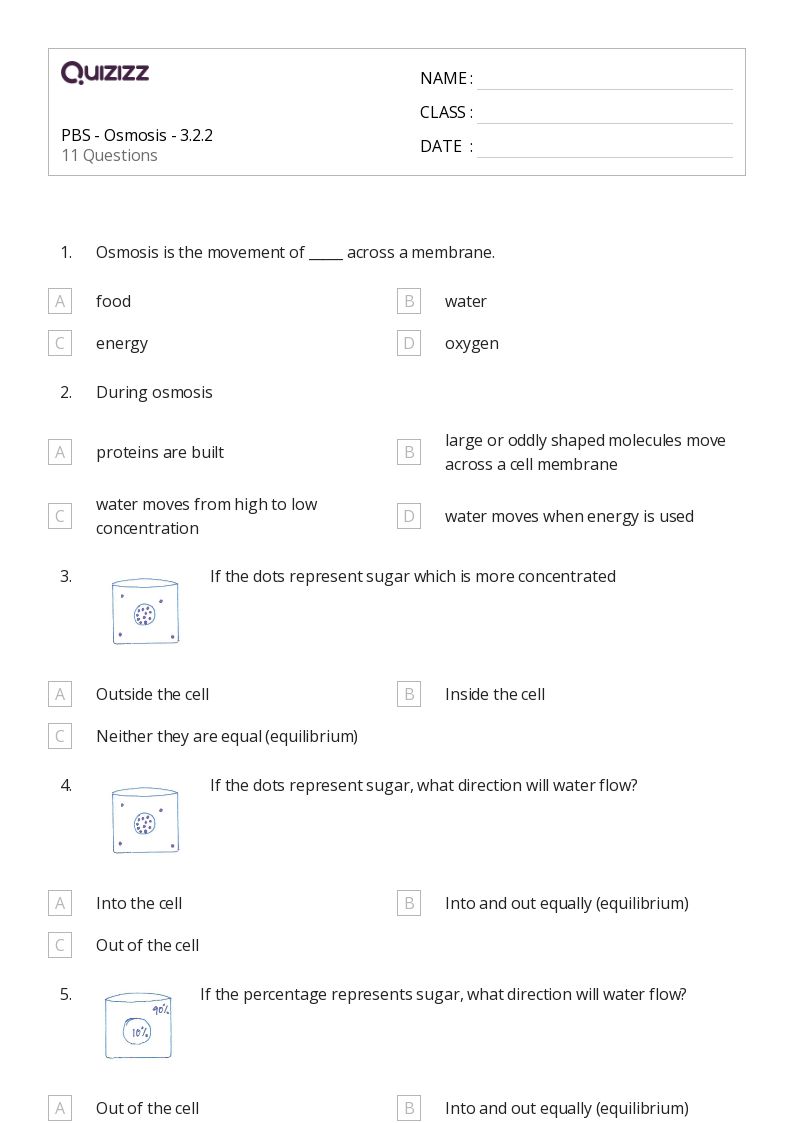
2. Dive into Detailed Worksheets


Worksheets provide structured problems that test your understanding:
- Concentration Gradient Exercises: Solve problems where you must calculate and predict the direction of particle movement.
- Membrane Permeability: Work through scenarios where the permeability of membranes affects osmosis rates.
- Facilitated Diffusion: Understand how specific channels or proteins can aid in the diffusion process.
📝 Note: Always check your work against provided solutions or use textbooks to confirm your answers.
3. Engage in Group Study Sessions


Discussing and solving problems with peers can greatly enhance your learning:
- Peer Teaching: Explain concepts to others, reinforcing your own understanding.
- Diverse Perspectives: Learn from different viewpoints on how to approach worksheet questions.
- Group Problem-Solving: Tackle complex problems together, dividing tasks for efficiency.
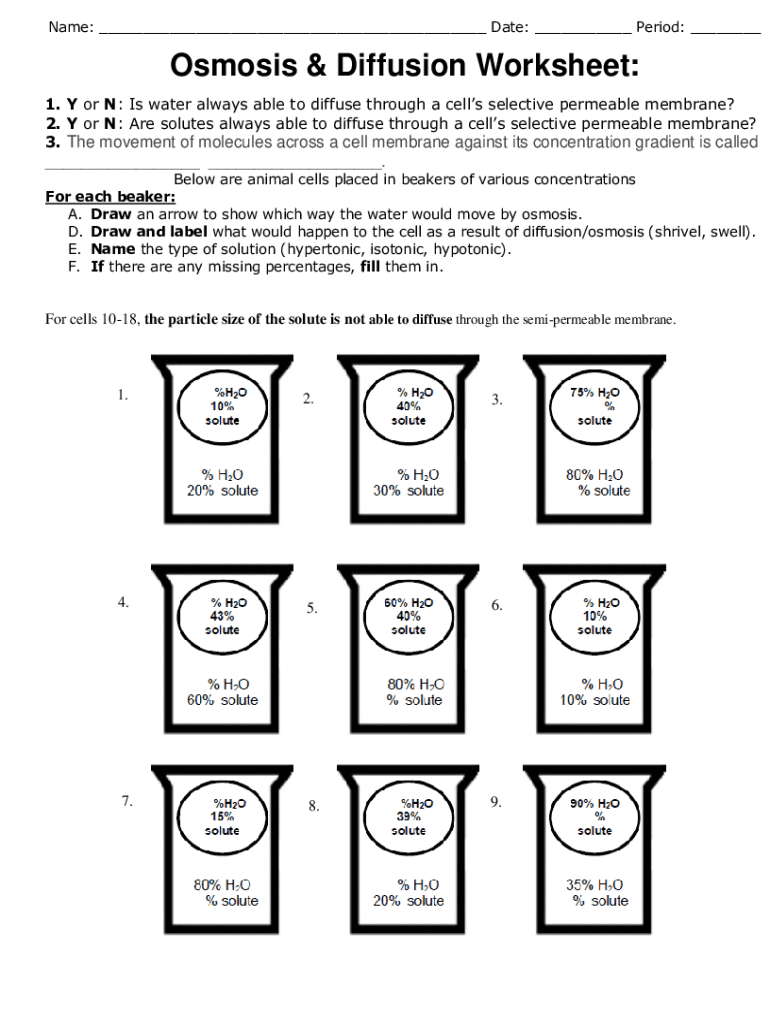
4. Use Real-Life Examples


Relating theoretical knowledge to everyday phenomena helps solidify concepts:
- Food Preservation: Understand how osmosis is used to preserve pickles through salt concentration.
- Biological Processes: Look at how osmotic pressure affects plant cells, causing turgor or plasmolysis.
- Health and Medicine: Explore the role of diffusion in kidney function or osmotic drug delivery.
| Example | Diffusion/Osmosis | Explanation |
|---|---|---|
| Pickling | Osmosis | Water leaves the cucumber cells into the salty brine, preserving the pickle. |
| Tea Steeping | Diffusion | Tea components dissolve and spread through hot water, driven by concentration differences. |
5. Review, Reflect, and Revise


Continuous improvement is key to mastering any subject:
- Review: Go over past worksheets and any mistakes to understand where you went wrong.
- Reflect: Consider how different factors impact diffusion and osmosis through real-life examples or case studies.
- Revise: Use past quiz or test answers to guide your study sessions, focusing on areas where you’ve made errors or found concepts challenging.
In wrapping up your journey with diffusion and osmosis worksheets, remember that the key to mastering these topics lies in a mix of hands-on learning, peer collaboration, real-world application, and reflective review. Whether you're visualizing the process through simulations, solving problems, or discussing with peers, each method enhances your understanding in unique ways. By consistently applying these strategies, you'll not only master the worksheets but also gain a deep appreciation for how these fundamental principles operate in living systems, agriculture, and even medical treatments. Let this knowledge guide you towards a broader and more profound understanding of biological systems and processes.
Why are diffusion and osmosis important to understand?
+
They are fundamental processes that govern how nutrients are transported in living organisms, how cells maintain homeostasis, and are crucial in various industrial applications like water purification.
Can diffusion and osmosis occur without a membrane?

+
Diffusion can happen without a membrane, but osmosis, by definition, involves the movement of water through a selectively permeable membrane due to concentration differences.
How can I better understand osmotic pressure?

+
Understanding osmotic pressure can be aided by visualizing it as the pressure exerted when water moves into an area of higher solute concentration, causing pressure to build up against the membrane.
What’s the difference between facilitated and simple diffusion?

+
Simple diffusion is the direct movement of particles down their concentration gradient, while facilitated diffusion uses transport proteins or channels to help substances move across the membrane.