Diffusion and Osmosis Beaker Worksheet: Answer Key Revealed

Understanding Diffusion and Osmosis in Beaker Experiments

In the realm of biology, chemistry, and even everyday life, understanding the processes of diffusion and osmosis is essential for comprehending how substances move within and between environments. This detailed blog post will explore these fundamental concepts through the lens of beaker experiments, offering insights into how they work, why they're important, and how you can apply this knowledge practically in educational settings or scientific research.
The Basics of Diffusion
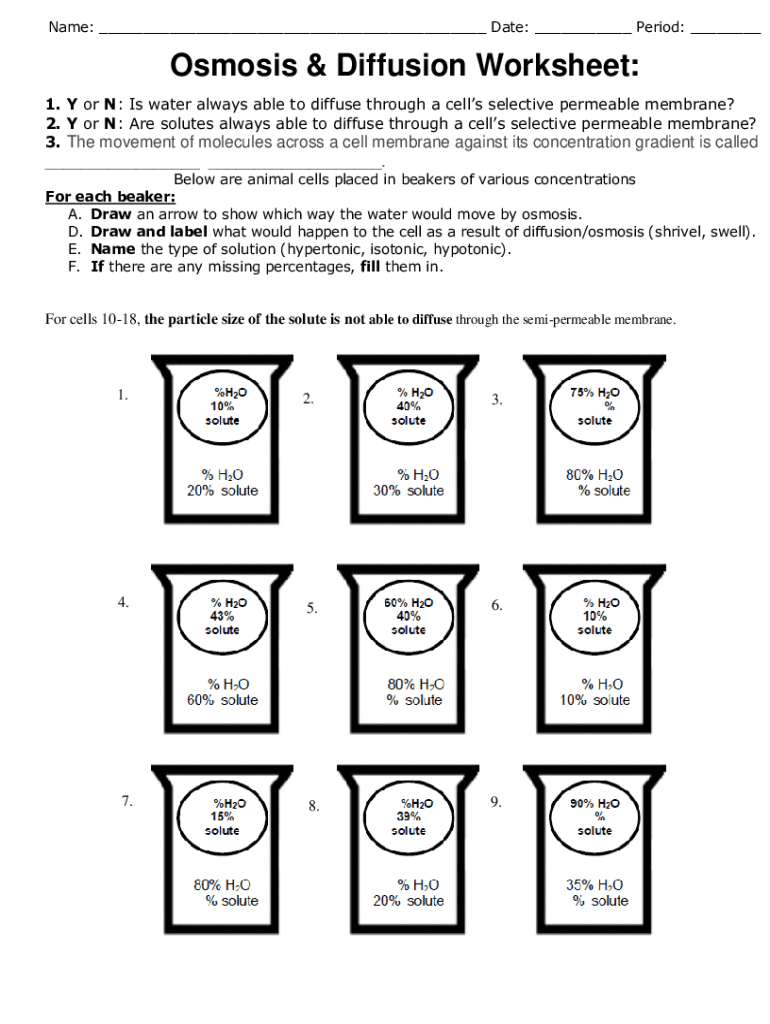
Diffusion is the natural movement of particles from an area of higher concentration to an area of lower concentration. Here's a breakdown of diffusion in a beaker experiment:
- Setup: Place a beaker with a dye or solute in a portion of water, but not all of it.
- Process: Over time, the dye will spread evenly throughout the beaker as diffusion occurs.
- Significance: This illustrates how molecules move in biological systems, like oxygen entering our lungs or nutrients spreading through the soil.
Here is how you might observe diffusion:
| Time | Observation |
|---|---|
| 0 Minutes | Color only in the bottom of the beaker |
| 5 Minutes | Color starts to spread upwards |
| 30 Minutes | Color evenly distributed |

The Process of Osmosis
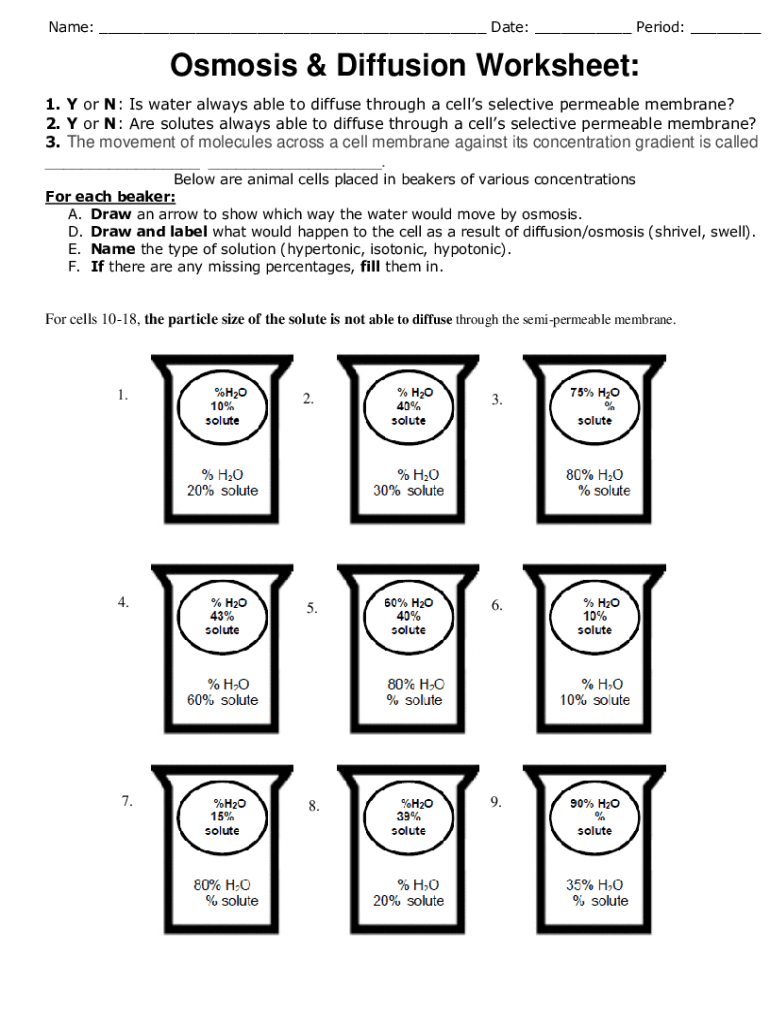
Osmosis is a specific type of diffusion involving the movement of water molecules across a semipermeable membrane, from a region of lower solute concentration to higher solute concentration.
- Setup: Place two beakers with a semipermeable membrane in between, one with pure water and the other with a concentrated solute.
- Process: Water molecules move towards the beaker with the solute, balancing out the concentration.
- Significance: This mimics how cells manage water balance, crucial for maintaining turgidity, preventing dehydration, or adjusting to osmotic pressure changes.
Applying Beaker Experiments in Education

Beaker experiments are an excellent teaching tool because:
- They visually demonstrate complex concepts like diffusion and osmosis.
- Students can measure and record observations, fostering scientific inquiry skills.
- These experiments can be modified for different levels of complexity, from basic elementary school projects to advanced research.
Important Notes

🧪 Note: Ensure proper handling of dyes or solutes; some might be toxic or reactive, so follow safety guidelines.
🔍 Note: Always use a control in experiments to have a valid comparison for your results.
Experimental Analysis

Analyzing results from beaker experiments can give insights into:
- Rate of Diffusion: How quickly does the substance spread? This can be influenced by temperature, size of the particles, and the medium.
- Osmotic Potential: The pressure exerted by water moving into a solution; this can be directly observed in beaker setups.
- Equilibrium: The point where concentrations balance out, and movement of particles occurs equally in both directions.
In the conclusion, it is crucial to recognize the practical applications of understanding diffusion and osmosis. From culinary arts to medical treatments, these processes are fundamental. Diffusion aids in the blending of flavors in cooking, while osmosis is used in kidney dialysis. These concepts not only enrich our understanding of biological systems but also have real-world applications that affect daily life. By exploring these phenomena through simple beaker experiments, we not only learn scientific principles but also develop an appreciation for the intricate balance of nature.
Why is understanding diffusion important for cooking?

+
Diffusion ensures flavors are evenly distributed throughout the food. For instance, marinating meat involves diffusion of flavors into the meat, enhancing taste and tenderness.
How does osmosis affect plant cells?
+
Osmosis helps plants maintain turgor pressure, which keeps cells rigid. This is crucial for plant structure; without it, plants would wilt.
Can osmosis be used to purify water?

+
Yes, reverse osmosis is a technique where water is forced through a membrane against the osmotic flow to remove impurities, providing clean drinking water.



