5 Steps to Solve Empirical and Molecular Formulas

Understanding and solving for empirical and molecular formulas is fundamental in chemistry, especially when you're looking to characterize compounds through analytical methods. Whether you're a student studying for your next chemistry exam or a researcher trying to understand the composition of a new material, mastering these calculations can significantly enhance your skills. In this article, we'll break down the five key steps needed to solve for empirical and molecular formulas.
Step 1: Gather the Data
Before you can dive into calculations, you need to have the right data:
- Percent composition of elements in the compound. This can come from experimental data or a given problem.
- If determining the molecular formula, you’ll also need the molar mass of the compound.
🔍 Note: Experimental data might come in the form of percent by weight, gram quantities, or mass ratios. Be sure to convert these into a format usable for your calculations.
Step 2: Calculate Mole Ratios
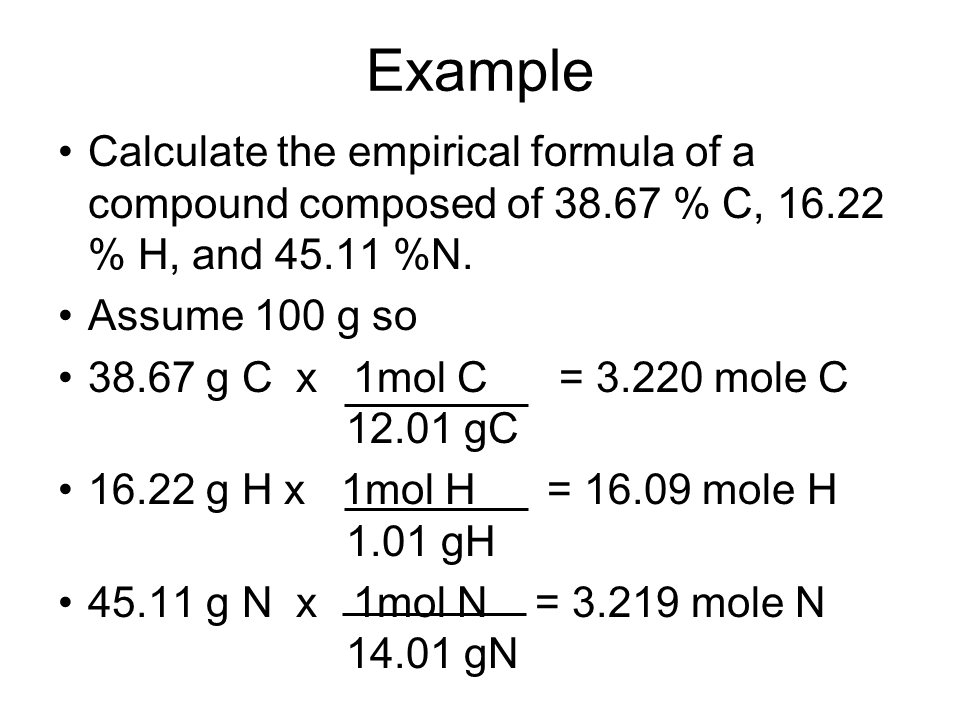
To find the empirical formula, you’ll need to determine the simplest whole number ratio of the atoms present in the compound. Here’s how:
- Convert percentages to grams: Assume you have 100 grams of the compound, making percentages equal to grams.
- Convert grams to moles: Use the atomic mass from the periodic table to convert grams of each element into moles. The formula is: Moles = (mass of element / atomic mass).
- Divide each mole value by the smallest mole value to get a ratio.
If the ratio isn’t a whole number, you might need to multiply everything by a common factor to get whole numbers.
Step 3: Determine the Empirical Formula
The empirical formula is the simplest whole number ratio of elements in a compound. From your calculated mole ratios, determine the subscripts for each element in the formula:
- Round the ratios to the nearest whole number, if necessary.
- If a ratio ends up being 0.5 or some other fraction, you’ll need to double all the numbers to get whole numbers.
📝 Note: For example, if you have a ratio of 1.5:1 for Carbon:Chlorine, you would double both values to get C:Cl = 3:2. This then forms the empirical formula, C3H2Cl.
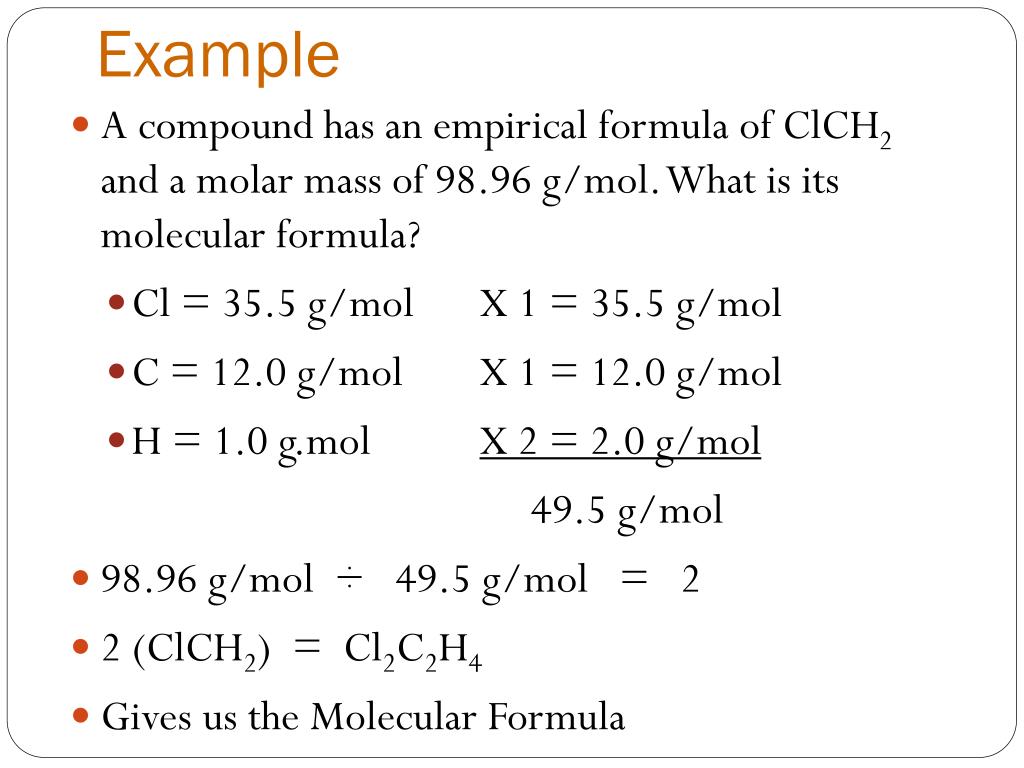
Step 4: Convert Empirical to Molecular Formula

If you’re given the molar mass of the compound, you can find the molecular formula:
- Calculate the molar mass of the empirical formula.
- Divide the molar mass of the compound by the molar mass of the empirical formula to get the multiplication factor.
- Multiply each subscript in the empirical formula by this factor to get the molecular formula.
Step 5: Double-Check Your Work

After your calculations:
- Recheck your percentages or data input.
- Verify that your empirical formula matches the molecular formula if you have the molar mass.
- Ensure the subscripts are whole numbers or in their simplest form.
Here’s a table summarizing these steps:
| Step | Action |
|---|---|
| 1 | Gather the data (percent composition or weight in grams, molar mass if needed). |
| 2 | Calculate mole ratios from grams to moles. |
| 3 | Determine the empirical formula using the simplest whole number ratio. |
| 4 | If molar mass is known, convert empirical to molecular formula. |
| 5 | Double-check calculations for accuracy. |
Mastering empirical and molecular formula calculations not only aids in understanding the structure of chemical compounds but also enhances your analytical skills in chemistry. By following these structured steps, you ensure that you're not just solving problems but learning the underlying principles that govern chemical composition. Remember, practice is key to perfecting these techniques, so continue experimenting with different compounds to solidify your knowledge.
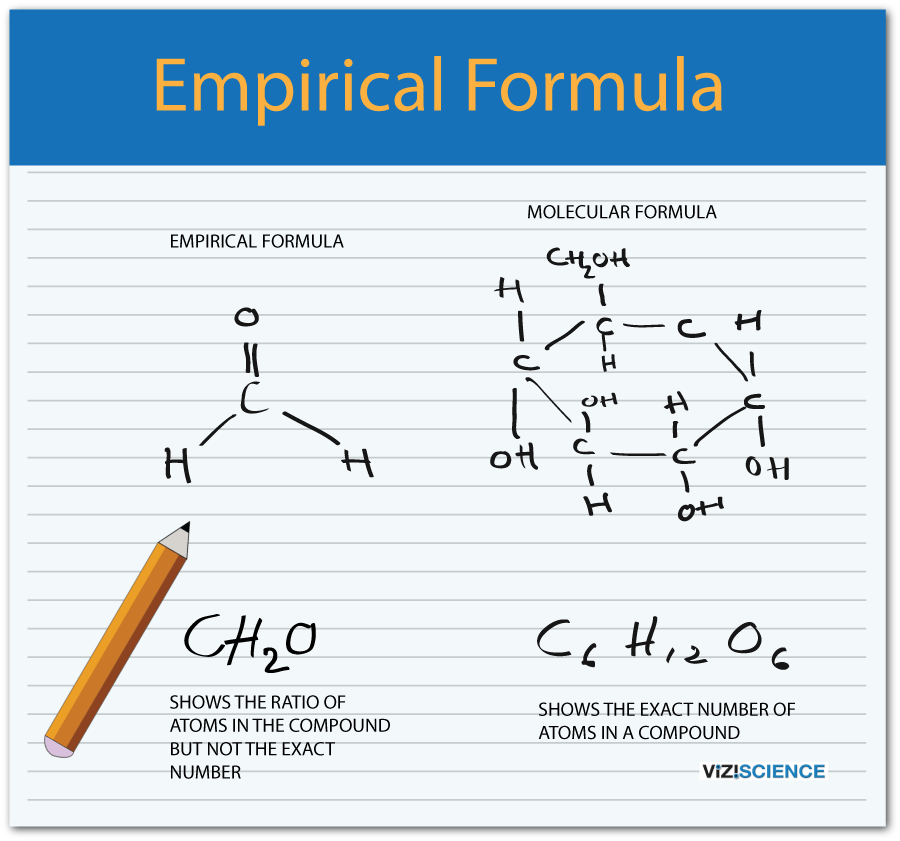
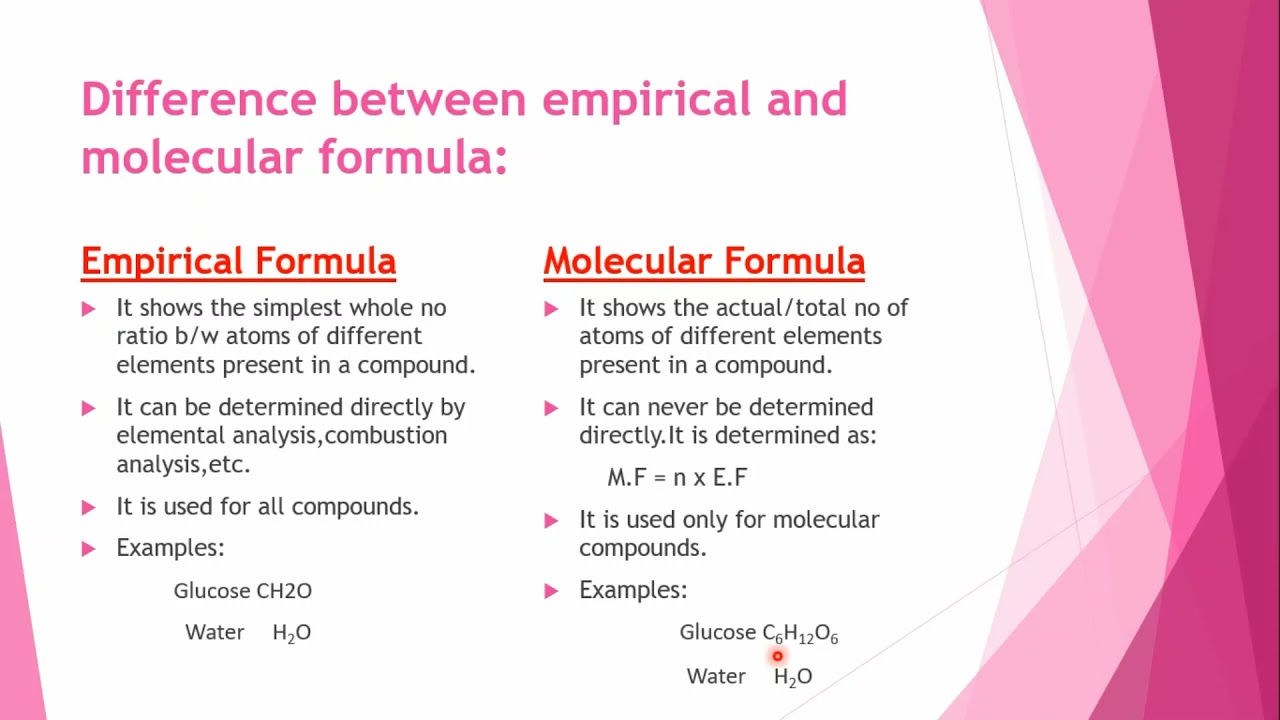
What is the difference between an empirical and a molecular formula?

+
An empirical formula shows the simplest whole number ratio of the atoms present in a compound, whereas a molecular formula reflects the actual number of atoms in one molecule of the compound.
How do I convert a mass percent to moles?

+
First, assume you have 100 grams of the compound, so mass percent equals mass in grams. Then, divide this by the element’s atomic mass to get moles.
Why might I get non-whole numbers when calculating mole ratios?

+
This often happens when the empirical formula is not the same as the molecular formula or if the sample isn’t pure. You might need to multiply all ratios by the same factor to get whole numbers.