5 Essential Tips for Solving Density Problems

Understanding Density
When it comes to understanding and solving density problems, whether for academic purposes or real-world applications, mastering the concept is key. Density is defined as the mass per unit volume of a substance and is an essential parameter in physics, chemistry, and various other fields. This blog post will explore five essential tips to help you tackle density problems effectively, ensuring you can understand, calculate, and apply this fundamental concept with confidence.
Tip 1: Know the Basic Formula

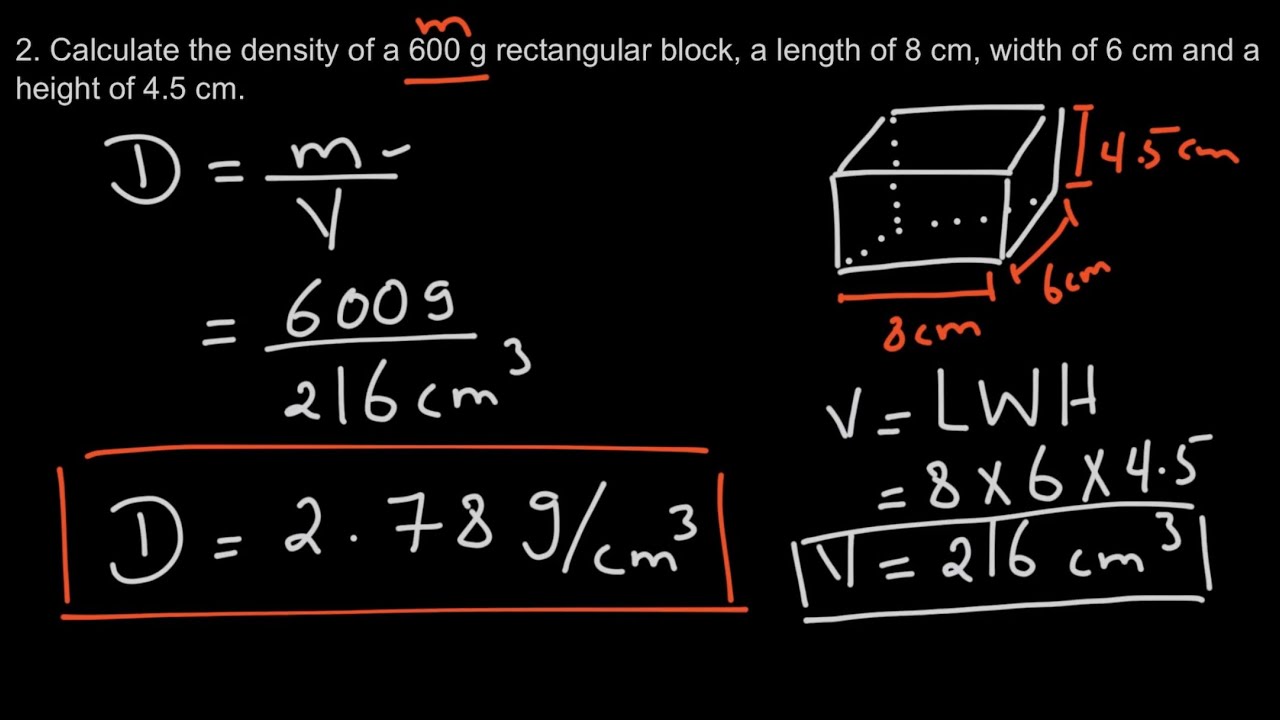
Density (d) is calculated using the formula:
d = m / V
- d: Density, measured typically in grams per cubic centimeter (g/cm³) or kilograms per liter (kg/L).
- m: Mass of the substance, usually in grams (g) or kilograms (kg).
- V: Volume, in cubic centimeters (cm³), milliliters (ml), or liters (L).
Remembering this formula is fundamental as it forms the basis for all density calculations. Here are some examples:
| Material | Density (g/cm³) | Mass (g) | Volume (cm³) |
|---|---|---|---|
| Water | 1.00 | 100 | 100 |
| Gold | 19.32 | 193.2 | 10 |
| Aluminum | 2.70 | 27.0 | 10 |
🔍 Note: The formula d = m/V is universal, but remember to check your units to ensure accuracy in your calculations!
Tip 2: Mastering Volume Calculation
Volume calculation can vary depending on the shape of the object:
- Rectangular Prism: V = Length × Width × Height
- Cube: V = Side³
- Cylinder: V = π × r² × h
- Sphere: V = (4/3) × π × r³
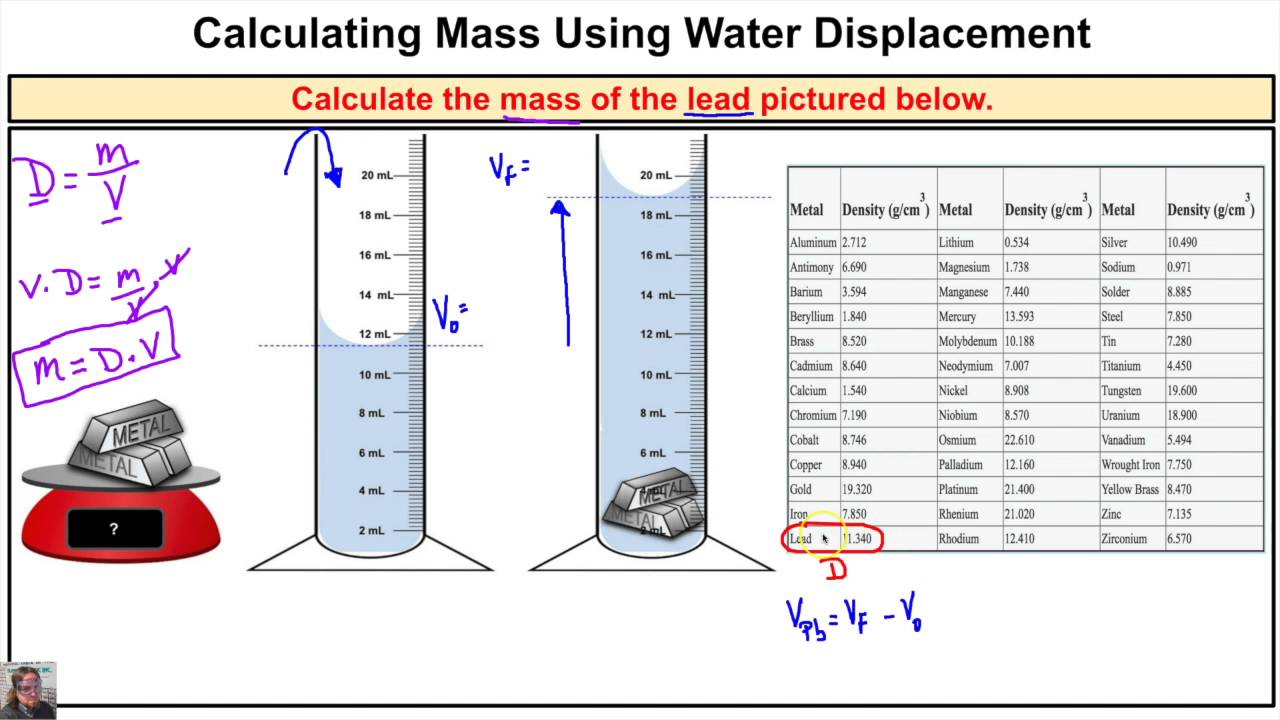
Here’s a trick to quickly calculate the volume of an irregular shape:
- Submerge the object in water, measure the rise in water level. This increase represents the object's volume.
Tip 3: Understanding Different Units

Density can be expressed in a variety of units, which can sometimes be confusing:
- kg/L
- g/mL
- g/cm³
- lbs/gal (imperial units)
To convert between these units, remember:
- 1 kg/L = 1000 g/L
- 1 g/mL = 1 g/cm³
- To convert lbs/gal to kg/L, use the conversion factor: 1 lbs/gal ≈ 0.1198 kg/L.
💡 Note: Always ensure your units match before performing calculations. Convert if necessary.
Tip 4: Use of Known Densities
Knowing the densities of common substances can help in solving problems faster:
| Substance | Density (g/cm³) |
|---|---|
| Water | 1.00 |
| Gold | 19.32 |
| Iron | 7.87 |
| Wood (Pine) | 0.51 |
Using known densities, you can:
- Quickly estimate the mass or volume of an object when one is known.
- Identify unknown materials by comparing their densities with known values.
Tip 5: Practical Applications and Problems

Density is not just theoretical; it has practical implications:
- Floatation: If an object’s density is less than the fluid it's in, it will float.
- Construction: The density of materials affects structural stability.
- Medicine: Bone density scans use density to diagnose conditions like osteoporosis.
Here's an example of how to solve a practical density problem:
Problem: You have a piece of metal weighing 500g with a volume of 50cm³. What is its density?
- Using the density formula:
- d = m / V = 500g / 50cm³ = 10 g/cm³
By applying these tips, you can navigate through density-related challenges with ease, whether it’s in school, work, or personal projects.
What units are typically used for density?

+
The most common units for density are grams per cubic centimeter (g/cm³) for solids, kilograms per liter (kg/L) for liquids, and pounds per gallon (lbs/gal) in imperial systems.
How can I find the volume of an irregular object?

+
You can use the water displacement method. Submerge the object in a known volume of water and measure how much the water level rises. The rise in water level represents the volume of the object.
Why does density matter in real life?

+
Density affects many aspects of daily life, from the buoyancy of objects in water, to the structural integrity of materials, to the diagnosis of health conditions like osteoporosis.
Can an object float if it’s denser than water?
+
No, an object will sink in water if its density is greater than that of water, as per Archimedes’ principle, which states an object will float if its density is less than the fluid it is in.
What if I have density in one unit but need it in another?

+
You can convert density units by understanding the relationships between mass and volume units. For example, to convert from g/mL to kg/L, you divide by 1000 because 1 kg = 1000 g.