Unlock the Secrets of Uranium-238 Decay with Our Worksheet Answers
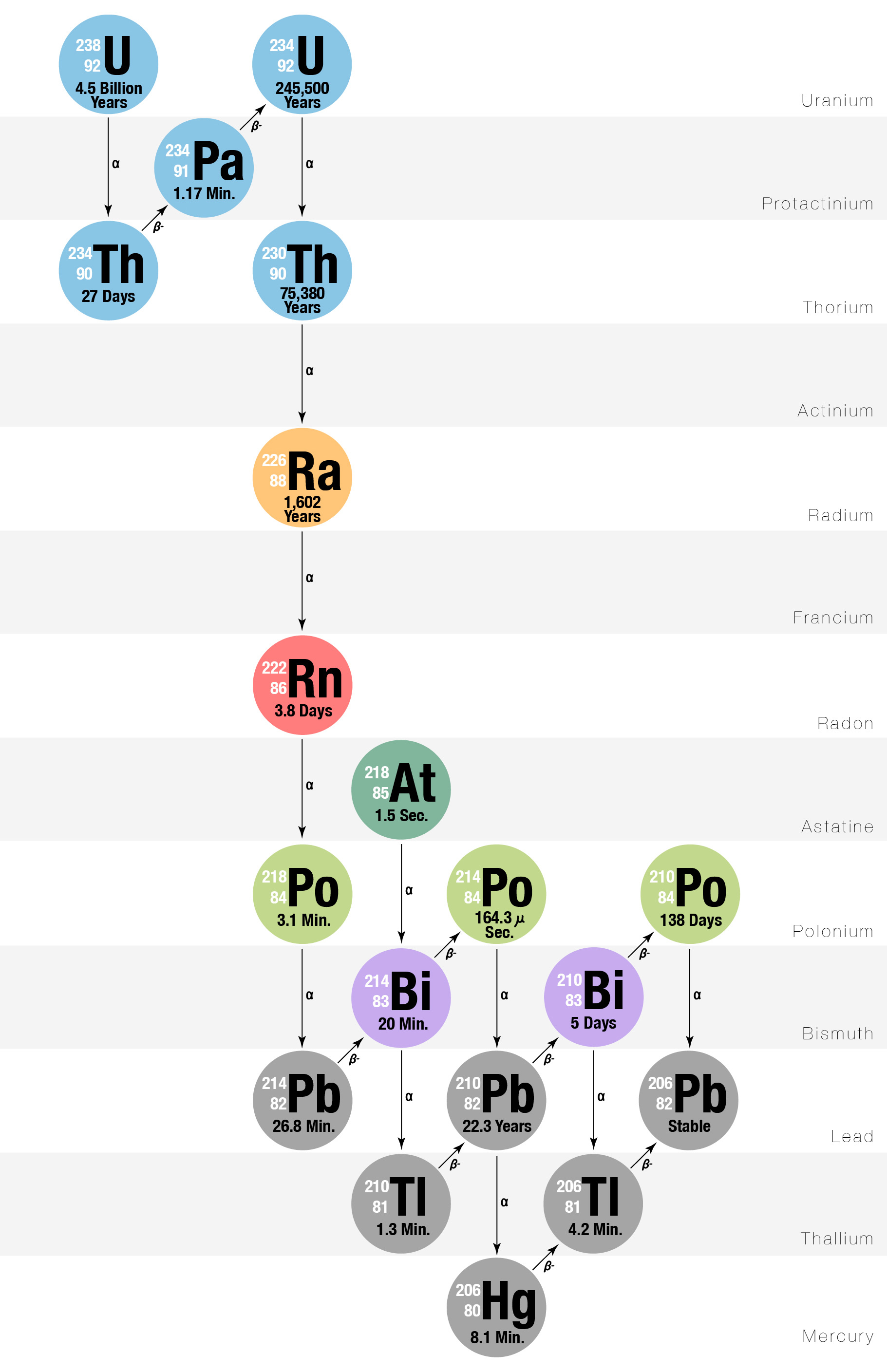
The fascinating world of nuclear physics presents itself in various forms, with the decay of Uranium-238 (U-238) being one of the most intriguing processes. This natural radioactive isotope of uranium undergoes a series of decay stages over its half-life of approximately 4.5 billion years, making it a fundamental study in the understanding of radioactivity and atomic stability. For students and enthusiasts looking to delve into this topic, let’s explore the secrets of Uranium-238 decay with the help of our comprehensive worksheet answers.
Understanding Uranium-238 Decay

U-238 decays through a complex chain known as the decay series of Uranium-238. This series involves a cascade of alpha and beta decays, transforming U-238 into a number of daughter nuclides before finally reaching stability as Lead-206 (Pb-206).
- Alpha Decay: In this process, U-238 emits an alpha particle, which consists of two protons and two neutrons, reducing its atomic mass by four units.
- Beta Decay: Following alpha emissions, some nuclides undergo beta decay, where a neutron turns into a proton, or vice versa, altering the atomic number.
Decay Series Overview

The decay series from U-238 to Pb-206 can be simplified as follows:
| Nuclide | Type of Decay | Half-life | End Product |
|---|---|---|---|
| Uranium-238 (U-238) | Alpha | 4.5 billion years | Thorium-234 |
| Thorium-234 (Th-234) | Beta | 24.1 days | Protactinium-234m |
| Protactinium-234m (Pa-234m) | Beta | 1.17 minutes | Uranium-234 |
| (…) | |||
| Lead-206 (Pb-206) | Stable | ||

🎓 Note: This table simplifies the complex decay series to include only a few key transformations.
Worksheet Analysis

Analyzing a worksheet on U-238 decay involves understanding the following key aspects:
- Decay Calculation: One can determine the time taken for a given mass of U-238 to decay into other isotopes using the half-life formula. The formula is:
N(t) = N₀ * (0.5)^(t/t₁₂)
Where:
- N(t) is the amount of the substance at time t
- N₀ is the initial amount of the substance
- t is the time that has elapsed
- t₁₂ is the half-life of the substance
Step-by-Step Decay Calculation Example

- Calculate the number of half-lives: Divide the time span by the half-life of U-238.
- Determine the remaining fraction: Using the formula above, calculate the fraction of U-238 that remains.
- Convert to mass: Multiply this fraction by the initial mass.
💡 Note: Make sure to use consistent units, typically grams or moles, throughout the calculation.
Practical Implications

The study of U-238 decay is not just an academic exercise:
- Geological Dating: U-238 decay is used to date geological formations, providing insights into the earth’s history.
- Environmental Impact: Understanding how uranium decays helps in assessing the environmental effects of nuclear processes.
- Nuclear Energy: The decay products of U-238 are relevant to the nuclear fuel cycle, particularly in the context of spent fuel rods.
Decoding the intricacies of Uranium-238 decay through worksheets can be an enlightening experience, offering students a hands-on approach to comprehending radioactive decay, half-life calculations, and the practical applications of this process. As we've worked through the worksheet answers, we've seen how this naturally occurring isotope changes over time, moving through a series of transformations to reach stability.
The journey from U-238 to Pb-206 is not just a process of decay but an elegant dance of atomic physics. It informs us about the history of our planet, impacts environmental policies, and aids in energy generation. By exploring these answers, students gain a deeper understanding of nuclear physics and its profound impact on our world. This exploration not only enhances their academic knowledge but also prepares them for the real-world applications of these principles, sparking curiosity about the invisible forces that shape our universe.
What is Uranium-238 used for in real life?

+
Uranium-238 is primarily used as nuclear fuel in nuclear reactors, although it’s less fissile than Uranium-235. It’s also used in geological dating, as well as in military applications for producing depleted uranium, which is used in armor-piercing rounds.
Why is Uranium-238 considered a naturally radioactive isotope?

+
Uranium-238 is radioactive because it has an unstable nucleus, making it naturally prone to decay. This instability arises from its large number of protons and neutrons, leading to a higher energy state than would be stable.
How can one safely handle Uranium-238?

+
Handling Uranium-238 should be done with extreme care due to its radioactivity. Protective gear, like lead shielding, and adherence to strict safety protocols are necessary to minimize exposure.
Can Uranium-238 be used for generating electricity?

+
While Uranium-238 is not typically used directly for generating electricity, its decay product, Thorium-234, eventually leads to the formation of Uranium-234, which can be enriched into Uranium-235 for use in nuclear reactors.
How long does it take for Uranium-238 to become stable?

+
Uranium-238 undergoes a series of decays over billions of years to finally become stable Lead-206, with its half-life being about 4.5 billion years.