Dalton's Law Worksheet Answers: Understand Partial Pressures Easily

When we delve into the realm of gases, Dalton's Law of Partial Pressures is fundamental for understanding how different gases interact in mixtures. This law posits that each gas in a mixture exerts pressure as if it were alone in the container, contributing independently to the total pressure of the system. If you're studying Chemistry or need to grasp partial pressures for applications like scuba diving or meteorology, mastering Dalton's Law through practical examples is essential. Here's a comprehensive guide to understanding and applying Dalton's Law using worksheet answers, offering clarity to both students and enthusiasts.
Introduction to Dalton’s Law

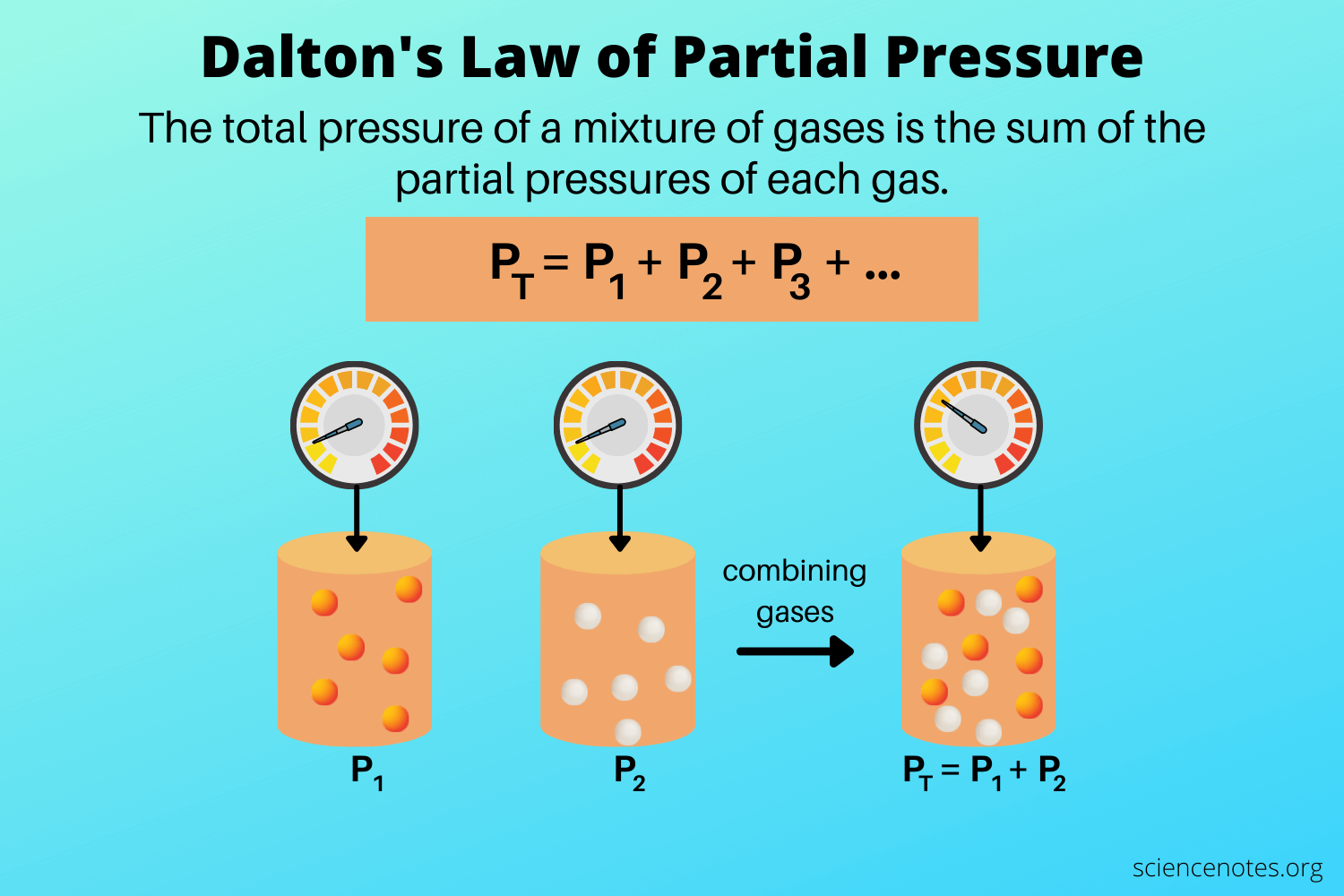
John Dalton, an English chemist and physicist, proposed this law in the early 19th century. The Law states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of individual gases.
Understanding Partial Pressure

Each gas in a mixture has its own contribution to the total pressure known as its partial pressure. Here’s how it works:
- Definition: The pressure each gas would exert if it alone occupied the volume of the mixture at the same temperature.
- Formula: Dalton’s Law is mathematically expressed as (P_{total} = P_1 + P_2 + … + P_n), where (P_1), (P_2), etc., are the partial pressures of each gas.
Worksheet Answers to Dalton’s Law Problems

To better grasp the concept, let’s delve into some examples and their solutions:
Example 1: Calculating Total Pressure

A container has 0.5 moles of oxygen and 0.8 moles of nitrogen at 298 K in a 10 L vessel. Calculate the total pressure.
- Step 1: Calculate partial pressures using the Ideal Gas Law ((PV = nRT)):
- Oxygen ((O_2)): (P_1 = \frac{(0.5)(0.0821)(298)}{10} = 1.23 \text{ atm})
- Nitrogen ((N_2)): (P2 = \frac{(0.8)(0.0821)(298)}{10} = 1.97 \text{ atm})
- Step 2: Sum the partial pressures to get the total pressure: (P{total} = 1.23 + 1.97 = 3.20 \text{ atm}).
🌍 Note: Temperature must be in Kelvin for these calculations.
Example 2: Determine Mole Fraction

A gas mixture has a total pressure of 5.0 atm, with nitrogen having a partial pressure of 3.5 atm. What is the mole fraction of nitrogen?
- Step 1: Mole fraction of a gas ((X)) is given by (\frac{P}{P{total}}):
- Step 2: (X{N_2} = \frac{3.5}{5.0} = 0.7).
Example 3: Applying Dalton’s Law to Scuba Diving

During a scuba dive, the partial pressure of nitrogen in the air tank must be controlled. Given a tank pressure of 200 atm and a desired nitrogen fraction of 0.79:
- Step 1: (P_{N_2} = 0.79 \times 200 = 158 \text{ atm}).
- Step 2: The total pressure will be slightly higher to account for other gases like oxygen.
Example 4: Gas Collection Over Water

Suppose hydrogen gas is collected over water at 25°C with a total pressure of 750 mmHg. Given the vapor pressure of water at 25°C is 23.8 mmHg:
- Step 1: (P_{H_2} = 750 \text{ mmHg} - 23.8 \text{ mmHg} = 726.2 \text{ mmHg}).
- Step 2: Use this pressure to find the volume or moles of (H_2) collected using the Ideal Gas Law.
🔬 Note: In experiments where gases are collected over water, the vapor pressure of water must be subtracted from the total pressure to find the pressure of the dry gas.
Applications of Dalton’s Law

Beyond academic applications, Dalton’s Law has practical uses:
- Meteorology: Understanding atmospheric pressure and humidity.
- Medical: Anesthesia delivery where precise gas mixtures are crucial.
- Industrial Applications: Gas storage and transport, air separation, and cryogenics.
In mastering Dalton's Law of Partial Pressures, you gain insight into not only how gases behave in mixtures but also how this behavior can be applied in various fields from science to everyday life. The worksheets and examples provided here illustrate how to navigate calculations involving partial pressures, enabling you to solve more complex problems with ease. As we have explored, understanding this law equips you with the tools to analyze gas behavior in different scenarios, offering a robust foundation for future learning and practical application.
Why is it important to consider the vapor pressure of water in gas collection experiments?

+
Water vapor exerts its own pressure, so not accounting for it can lead to inaccurate measurements of the pressure or volume of other gases being collected.
Can Dalton’s Law apply to gas mixtures in scuba diving?
+
Yes, it’s crucial to manage partial pressures to avoid conditions like nitrogen narcosis and oxygen toxicity.
What are the limitations of Dalton’s Law?

+
The law assumes ideal gas behavior, neglecting intermolecular forces which can be significant at high pressures or low temperatures.
How does Dalton’s Law relate to atmospheric pressure?

+
Atmospheric pressure is the sum of the partial pressures of all the gases present in the atmosphere, including nitrogen, oxygen, and other trace gases.



