Master Covalent Nomenclature with Our Handy Worksheet

Understanding and mastering covalent nomenclature is essential for students studying chemistry. Covalent compounds, formed by the sharing of electrons between atoms, come with their own set of naming rules which can often seem daunting. However, with the right approach and tools, the learning curve becomes much more manageable. In this blog post, we'll delve deep into the world of covalent nomenclature, explaining why it's crucial, how to name these compounds correctly, and providing a comprehensive worksheet to solidify your knowledge.
The Importance of Covalent Nomenclature

Before diving into the rules, let’s understand why mastering covalent nomenclature is critical:
- Communication in Science: Proper naming of compounds ensures clear communication among chemists worldwide. It eliminates confusion and facilitates the sharing of scientific discoveries.
- Chemical Formulas: Naming helps in determining the chemical formula from the name, aiding in understanding the composition of compounds.
- Preparation for Advanced Topics: It’s a foundational knowledge for learning more complex topics like organic chemistry, where covalent bonds play a significant role.
Key Rules for Naming Covalent Compounds
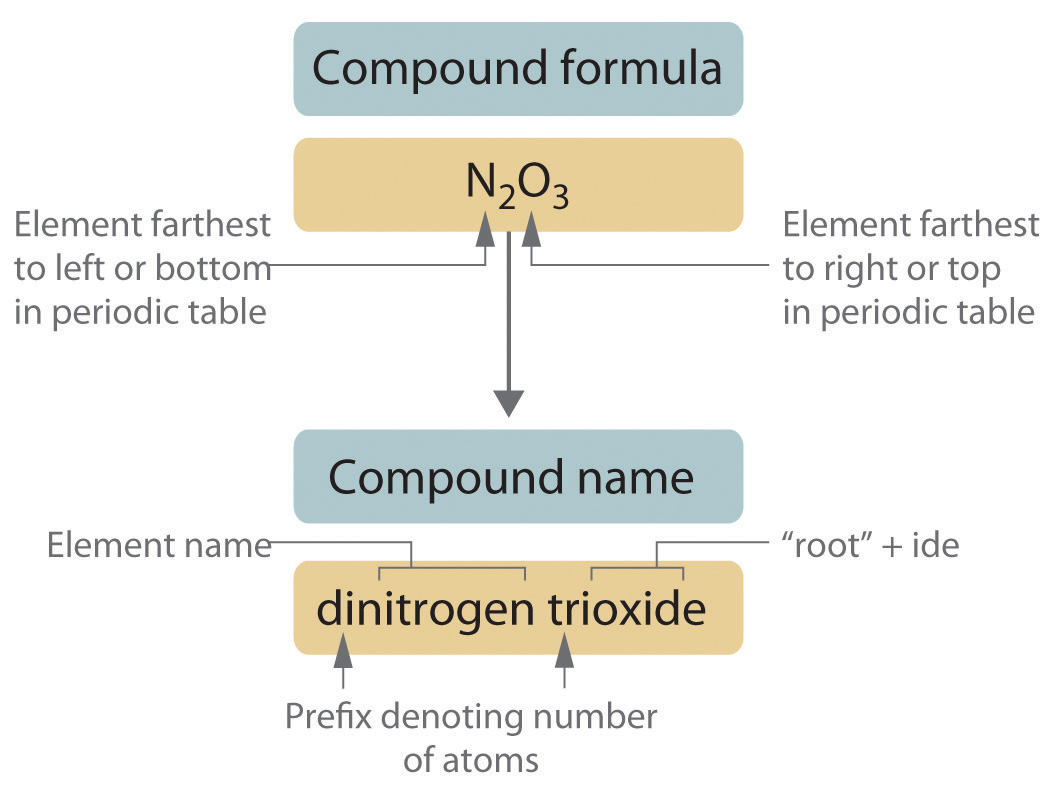
Here are the fundamental steps for naming binary covalent compounds:
- Identify the Elements: Start by recognizing the elements present in the compound.
- Assign Prefixes: Use Greek prefixes to indicate the number of atoms of each element. The prefixes are:
- mono- (1)
- di- (2)
- tri- (3)
- tetra- (4)
- penta- (5)
- hexa- (6)
- hepta- (7)
- octa- (8)
- nona- (9)
- deca- (10)
- Write the Name:
- Name the less electronegative element first, changing the ending of the second element to -ide.
- If only one atom of the first element is present, the prefix “mono-” is usually omitted.
- Drop any final ‘a’ or ‘o’ in the prefix if the element’s name begins with a vowel to avoid awkward pronunciation.
💡 Note: The most electronegative element always gets the -ide suffix, regardless of its position in the periodic table.
Putting It Into Practice: A Worksheet

Here’s a practical way to reinforce your understanding of covalent nomenclature:
Naming Covalent Compounds Worksheet

| Compound Formula | Element Name | Name of Compound |
|---|---|---|
| CO2 | Carbon, Oxygen | Carbon dioxide |
| N2O | Nitrogen, Oxygen | Dinitrogen monoxide |
| PCl3 | Phosphorus, Chlorine | Phosphorus trichloride |

Complete the following table with the correct names for each compound:
| Compound Formula | Name |
|---|---|
| CO | [Answer] |
| SO2 | [Answer] |
| CH4 | [Answer] |
| NF3 | [Answer] |
Final Thoughts

Mastering covalent nomenclature is not just about memorizing rules but understanding the logic behind how compounds are named. This knowledge allows you to predict properties based on the name alone, which is a powerful tool in chemistry. By practicing with worksheets, as we've provided, you reinforce your understanding and become fluent in a language that connects the dots in the vast field of chemistry.
This mastery not only aids in academic success but also in practical applications, from industrial processes to environmental science, where knowing how to correctly name compounds can lead to significant insights and innovations.
Why is the ending of the second element changed to -ide?

+
The -ide suffix denotes that the element is the more electronegative part of the compound, helping to maintain consistency and clarity in nomenclature.
What do I do if a compound has three elements?
+
For compounds with three elements, you use similar rules, but you might also incorporate prefixes or change the name of the polyatomic ion if present.
Can I skip the prefix ‘mono-’ when naming?

+
Yes, for the first element in the compound, ‘mono-’ is usually omitted unless there’s a need to distinguish between compounds with different numbers of that element.
How do you differentiate between different compounds?

+
By using different prefixes for the number of atoms and ensuring that the electronegativity rule is followed, you can name compounds correctly without confusion.