5 Ways to Master Conjugate Acid Base Pairs Easily

In the intricate world of chemistry, understanding how substances react with each other is pivotal, especially when it comes to acids and bases. One of the foundational concepts in this arena is the study of conjugate acid-base pairs. Mastering these pairs is not just about rote learning; it involves a deep understanding of the chemical behavior, reactions, and their significance in chemical equilibrium. Here, we delve into five efficient ways to understand and master conjugate acid-base pairs, making chemistry not just easier but also more fascinating.
1. Comprehend the Basics

Before diving into the complexities, ensure you have a firm grasp on what constitutes acids and bases:
- Acids are proton (H+) donors.
- Bases are proton (H+) acceptors.
When an acid donates its proton, it forms its conjugate base, and when a base accepts a proton, it becomes its conjugate acid. Understanding this dynamic is the first step in mastering conjugate pairs.
2. Utilize Visual Aids
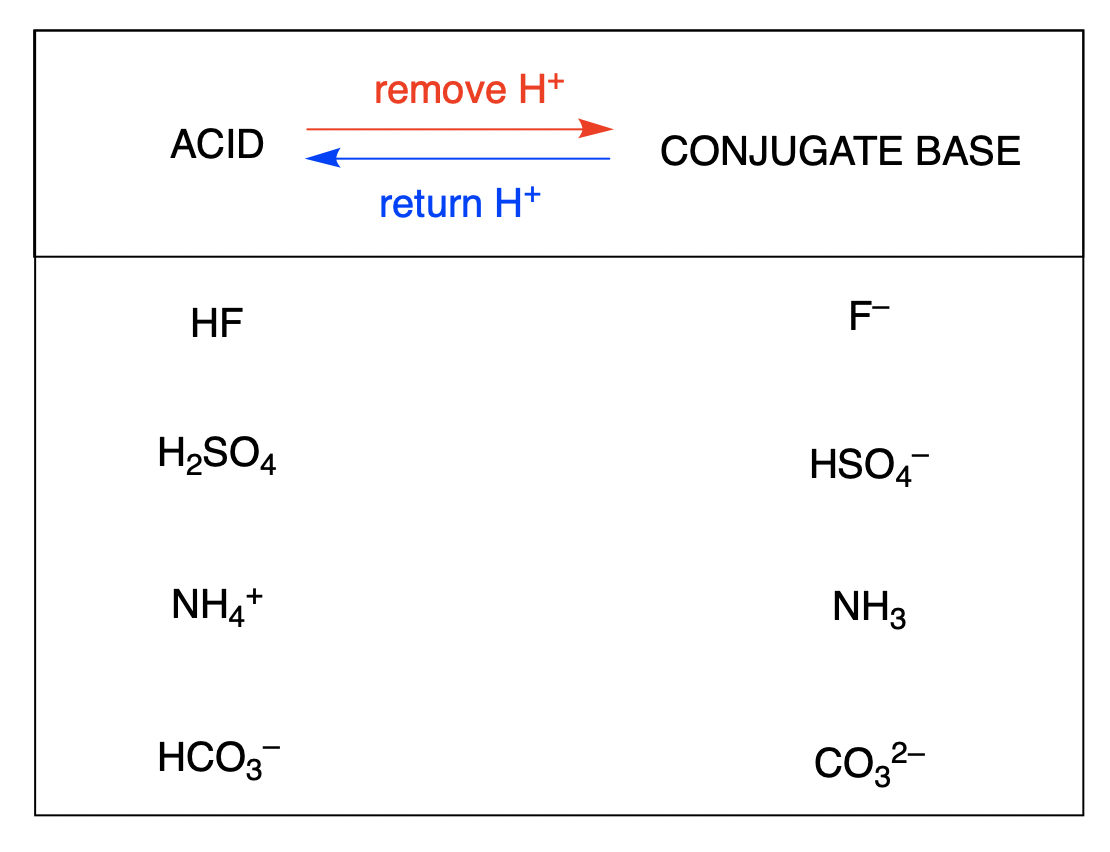
Chemistry can often be a visual science:
- Reaction Maps: Create diagrams or maps showing acids donating protons to form conjugate bases, and vice versa. This visual representation helps in understanding the balance of protons and charges.
- Charge Distribution: Use Lewis structures or electron dot formulas to see how protons are shared or donated, thus facilitating the visual understanding of acid-base reactions.
🔍 Note: Visual aids not only help in understanding but also make recalling these concepts during exams much easier.
3. Practice Identifying Conjugate Pairs
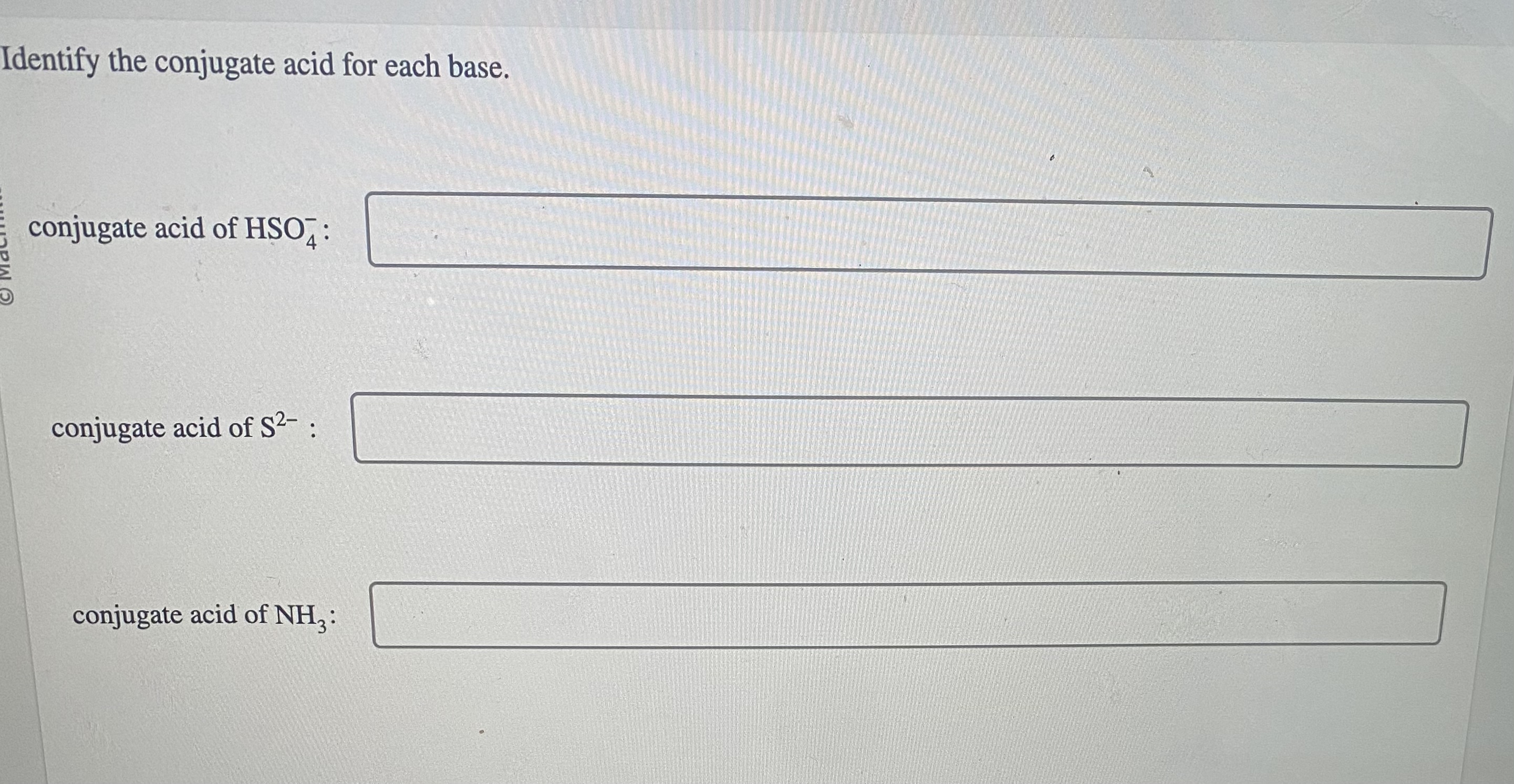
Engage in active learning by practicing:
- List out common acids and their conjugate bases.
- Go through acid-base reactions and identify the conjugate acid/base pairs in each.
- Use flashcards or apps designed for chemistry to test your knowledge regularly.
4. Understand the Brønsted-Lowry Theory

The Brønsted-Lowry theory expands the Arrhenius definitions by stating that acids donate H+ and bases accept H+. This theory:
- Allows for substances that do not contain hydroxide ions to be classified as bases.
- Shows the reversibility of acid-base reactions, making conjugate pairs more tangible in the context of equilibrium.
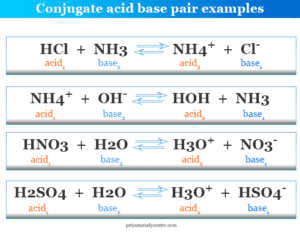
5. Relate to Real-Life Examples

Connect theoretical chemistry with real-life applications:
| Example | Conjugate Acid-Base Pair |
|---|---|
| Carbonic acid in beverages (carbonation) | H2CO3/HCO3- |
| Ammonia cleaners | NH3/NH4+ |
| Antacid tablets | CaCO3/HCO3- |
This correlation helps in understanding the practical importance of conjugate acid-base pairs in everyday life.
Concluding Thoughts

The mastery of conjugate acid-base pairs isn’t just about memorization; it’s about understanding the dynamic nature of chemical reactions and their equilibrium states. By grasping the basics, utilizing visual tools, practicing identification, understanding theories, and relating to real-life examples, you can not only excel in your chemistry studies but also appreciate the intricate balance that exists in chemical systems. Chemistry is at play in every sip of soda, every splash of cleaner, and every pill we take, making the study of conjugate acid-base pairs a compelling journey through our daily existence.
Why are conjugate acid-base pairs important in chemical reactions?

+
Conjugate acid-base pairs are crucial because they help understand the behavior of substances in solution, particularly in acid-base reactions. They are key in determining the direction of reactions, the strength of acids and bases, and equilibrium dynamics.
Can you give an example of an everyday use of conjugate acid-base pairs?

+
One common example is the use of antacids like Tums (CaCO3) to neutralize stomach acid (HCl). Here, CaCO3 acts as a base, and when it reacts with HCl, it forms HCO3-, the conjugate base, which then interacts with the stomach acid to provide relief from acidity.
How can I differentiate between acids and bases using their conjugate pairs?
+
The key is to look at what’s happening to the protons (H+). If a substance can donate a proton, it acts as an acid, and the species left after proton donation is its conjugate base. Conversely, if a substance can accept a proton, it acts as a base, and the species formed after accepting a proton is its conjugate acid.