Colligative Properties Answers: Worksheet Mastery Guide

In the vast realm of chemistry, understanding colligative properties is not just beneficial but essential for students and professionals alike. These properties emerge when solute particles affect a solvent's physical properties in a way that depends on the concentration of the solute rather than its chemical identity. This Worksheet Mastery Guide is designed to delve deep into colligative properties, providing comprehensive answers, practical examples, and insights that will equip learners to excel in their studies.
Introduction to Colligative Properties


Colligative properties refer to the changes in physical characteristics of a solution based on the quantity of solute present, rather than its nature. The four primary colligative properties are:
- Vapor Pressure Lowering
- Boiling Point Elevation
- Freezing Point Depression
- Osmotic Pressure
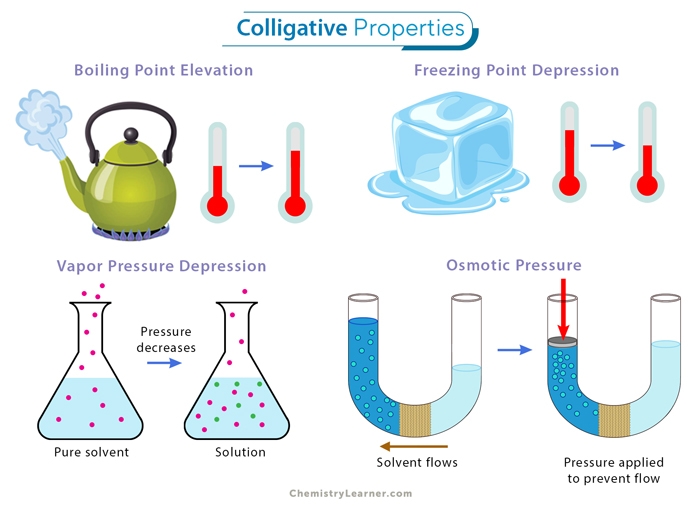
Vapor Pressure Lowering

The presence of non-volatile solutes reduces the vapor pressure of a liquid. This can be explained by:
- Decrease in the number of solvent molecules at the surface, which lowers the rate of evaporation.
- The solute occupies space where solvent molecules would typically evaporate.
The relationship between vapor pressure and solute concentration is given by Raoult’s Law:
Psolution = P°solvent * Xsolvent
where Psolution is the vapor pressure of the solution, P°solvent is the vapor pressure of pure solvent, and Xsolvent is the mole fraction of the solvent.
Boiling Point Elevation

When a solute is added to a solvent, the boiling point increases. This happens because the solute reduces the vapor pressure, and the solution must be heated to a higher temperature to achieve the necessary vapor pressure for boiling:
- ΔTb = i * Kb * m
Here, ΔTb is the boiling point elevation, i is the Van’t Hoff factor, Kb is the ebullioscopic constant, and m is the molality of the solution.
Freezing Point Depression

Similar to boiling point elevation, adding solute lowers the freezing point:
- ΔTf = i * Kf * m
Where ΔTf is the freezing point depression, i is the Van’t Hoff factor, Kf is the cryoscopic constant, and m is the molality.
Osmotic Pressure

This is the pressure required to prevent osmosis, which occurs when solvents move through a semi-permeable membrane to equalize solute concentration:
- π = MRT
Where π is osmotic pressure, M is the molarity of the solution, R is the gas constant, and T is the temperature in Kelvin.
Worksheet Examples and Answers

| Question | Answer |
|---|---|
| Calculate the vapor pressure lowering of a 0.5 M sucrose solution at 25°C. (P° of water at 25°C = 23.76 mmHg) | Using Raoult’s Law: Psolvent = 23.76 mmHg * 0.988 = 23.49 mmHg. Thus, the vapor pressure lowering is 23.76 mmHg - 23.49 mmHg = 0.27 mmHg. |
| Find the boiling point of a 1.5 m glucose solution in water. | ΔTb = (1 * 0.512°C/m * 1.5 m) = 0.768°C. Thus, the boiling point increases from 100°C to 100.768°C. |
| Determine the freezing point of a 0.5 m ethylene glycol solution in water. | ΔTf = (1 * 1.86°C/m * 0.5 m) = 0.93°C. So, the freezing point depresses from 0°C to -0.93°C. |
| Calculate the osmotic pressure of a 0.2 M NaCl solution at 298 K. | π = (2 * 0.2 M * 0.0821 L·atm/mol·K * 298 K) = 9.72 atm. |

Conclusion

As we wrap up this comprehensive guide to colligative properties, it’s clear that these properties are fundamental in understanding how solutions behave under different conditions. By mastering these concepts, students can not only answer worksheet questions with confidence but also apply this knowledge in practical scenarios like antifreeze formulation, molecular weight determination, and osmotic pressure regulation in biological systems. The key is to remember that these properties are governed by the quantity of solute particles, not their identity, allowing for a wide range of applications in both academic and industrial contexts.
What are the differences between boiling point elevation and freezing point depression?

+
Boiling point elevation refers to an increase in the boiling point of a solvent due to the addition of a solute, while freezing point depression describes the decrease in the freezing point. Both phenomena occur because the solute particles interfere with the solvent’s natural processes of evaporation and freezing.
How do ionic solutes affect colligative properties?
+
Ionic solutes like salts dissociate into multiple particles (ions) in solution, thus increasing the effective number of solute particles. This leads to greater changes in the colligative properties as per the Van’t Hoff factor.
Can colligative properties be used to determine the molecular weight of an unknown compound?
+
Yes, by measuring how a known amount of the compound affects the boiling or freezing point of a solvent, one can calculate its molecular weight using the respective equations for boiling point elevation and freezing point depression.