Chemistry Unit 5 Worksheet 2: Master Molecules Now

Are you ready to delve deeper into the fascinating world of chemistry? Whether you're a student grappling with the basics or a seasoned chemist seeking a refresher, understanding molecular structure and chemical bonding is fundamental. In this comprehensive guide, we'll explore key concepts from Chemistry Unit 5 Worksheet 2, designed to help you master molecules once and for all.
Understanding Molecules and Their Bonds

At the core of chemistry lies the molecule, a stable particle composed of two or more atoms held together by chemical bonds. Here's what you need to grasp:
- Covalent Bonds: Formed by sharing electrons, these bonds allow atoms to achieve a stable electron configuration.
- Ionic Bonds: Occur when electrons are transferred from one atom to another, creating oppositely charged ions that attract each other.
- Hydrogen Bonds: Not a true bond, but a strong interaction between polar molecules containing hydrogen.
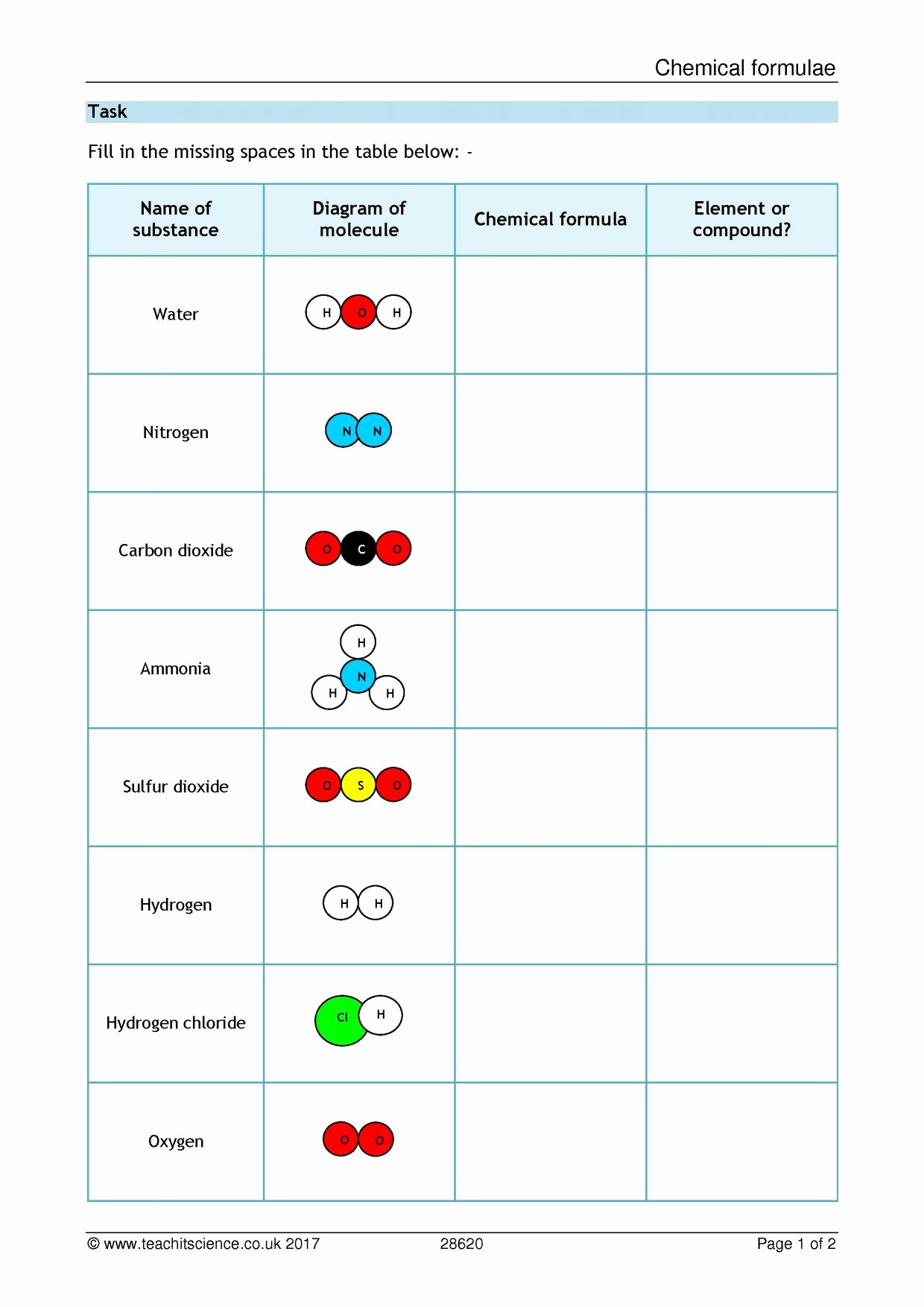
To better visualize, let's look at the following table:
| Bond Type | Description | Examples |
|---|---|---|
| Covalent | Sharing of electrons between atoms. | H2O (Water), CO2 (Carbon Dioxide) |
| Ionic | Transfer of electrons resulting in charged ions. | NaCl (Table Salt) |
| Hydrogen | Attraction between hydrogen and electronegative atoms. | DNA base pairs, Water molecules |
Key Principles in Molecular Geometry

The shape of molecules significantly influences their chemical properties:
- VSEPR Theory: Valence Shell Electron Pair Repulsion Theory helps predict the geometry by considering electron pair repulsion.
- Hybridization: The concept where atomic orbitals mix to form new, hybrid orbitals suitable for bonding.
VSEPR Theory in Action
Here are some examples of how VSEPR theory applies to different molecules:
- Methane (CH4): Has a tetrahedral shape because the carbon atom has four bonding electron pairs.
- Ammonia (NH3): Exhibits a trigonal pyramidal shape due to the lone pair repulsion.
- Water (H2O): A bent shape arises from two lone pairs on the oxygen atom.
⚗️ Note: Understanding molecular geometry not only helps in visualizing molecules but also in predicting their reactivity and polarity.
Understanding Molecular Polarity

The distribution of charge in a molecule determines its polarity:
- Polar Molecules: Have an asymmetrical distribution of electrons, leading to a dipole moment.
- Non-Polar Molecules: Have symmetrical electron distribution or no polar bonds.
Determining Molecular Polarity

Here's how you can determine if a molecule is polar:
- Draw the molecule's Lewis structure.
- Assess the geometry using VSEPR theory.
- Determine if there are polar bonds by checking the electronegativity difference between atoms.
- If the molecule has polar bonds and an asymmetric shape, it's likely polar.
🔍 Note: Not all molecules with polar bonds are polar; symmetry can cancel out dipole moments, making the molecule non-polar overall.
Interactive Learning Tools

Visual aids and interactive simulations can be incredibly beneficial:
- Molecular Model Kits: Provide a hands-on approach to understanding spatial arrangement and bonding.
- Online Simulations: Use software to rotate and visualize molecules in 3D.
Tips for Using Learning Tools Effectively

- Start with simple molecules and work your way up to more complex ones.
- Practice building molecules and predicting their shapes and polarity.
🎨 Note: Experimenting with molecular models can enhance your understanding of how structure relates to function in chemistry.
Key Takeaways and Final Thoughts

As we conclude this journey through molecular structure and bonding, remember these key points:
- Molecular structure influences the properties of substances in chemistry.
- Understanding different types of bonds, molecular geometry, and polarity is crucial for mastering molecules.
- Utilizing educational tools can significantly enhance your learning experience and comprehension.
With a solid grasp of these fundamentals, you're now better equipped to tackle more complex chemical problems. Chemistry is a vast field, but mastering the basics is the key to unlocking further understanding and appreciation.
Why are covalent bonds important in organic chemistry?

+
Covalent bonds form the backbone of organic molecules, enabling complex molecular structures vital for life processes.
How does molecular shape affect a molecule’s reactivity?

+
The shape influences how molecules can interact with others, impacting reaction rates, catalysis, and specificity in biological systems.
What is the significance of hydrogen bonding?

+
Hydrogen bonds play critical roles in the properties of water, DNA structure, and protein folding, crucial for many biological functions.