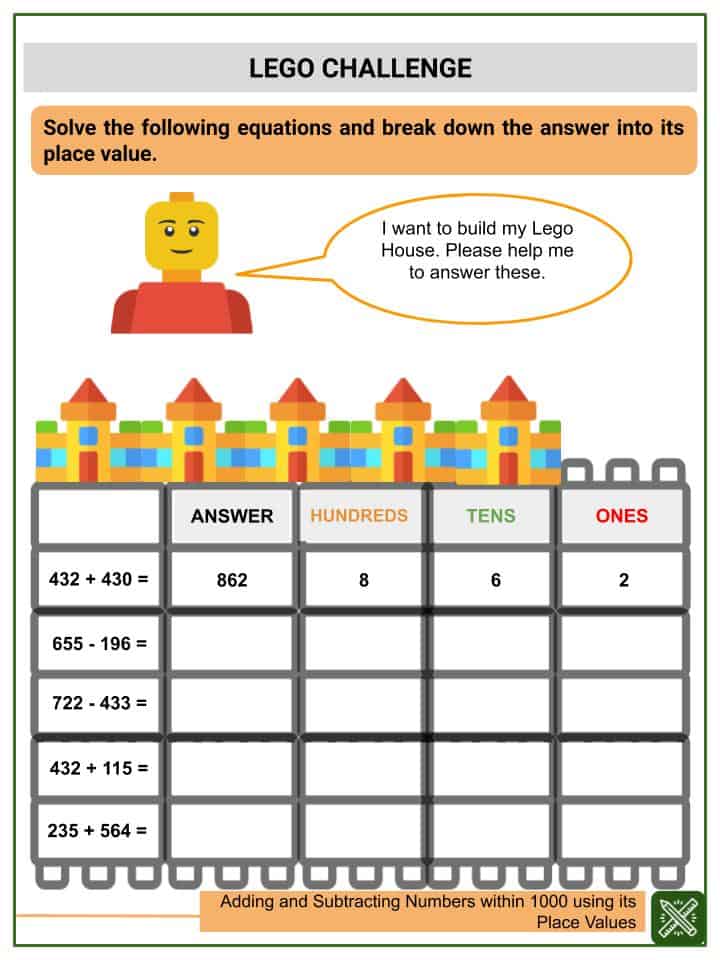
5 Essential Tips for Acing Chemistry Unit 4 Worksheet 2
The journey through high school chemistry can be daunting, especially when you reach topics like thermochemistry and equilibrium in Chemistry Unit 4. These concepts are not just abstract; they are fundamental in understanding many everyday processes and industrial applications. To help you master these topics, here are five essential tips to ace your Chemistry Unit 4 Worksheet 2:
1. Understand the Basics

Before diving into complex calculations and equilibrium concepts, ensure you have a solid foundation in the basics of chemistry:
- Stoichiometry: Know how to balance chemical equations and calculate mass relationships between reactants and products.
- Enthalpy and Energy: Understand the concepts of exothermic and endothermic reactions, and how to calculate enthalpy changes.
- Acid-Base Chemistry: Grasp the definitions of acids and bases according to Arrhenius, Bronsted-Lowry, and Lewis theories.
When you are well-versed in these foundational elements, solving problems in worksheet 2 will be less challenging as you’ll be able to apply these principles logically.
2. Focus on Key Formulas and Calculations

Chemistry Unit 4 often requires extensive use of formulas for calculating:
- Enthalpy Changes: Use ΔH = mcpΔT or Hess’s Law for complex reactions.
- Equilibrium Constants: Understand the relationships between Kc, Kp, Qc, and reaction direction.
- Rate Laws: Determine reaction orders, rate constants, and how concentrations affect reaction rates.
Create flashcards or use mnemonic devices to remember these formulas. Here’s a simple table to aid your memory:
| Concept | Formula |
|---|---|
| Enthalpy Change | ΔH = ΣΔHf(products) - ΣΔHf(reactants) |
| Equilibrium Constant | Kc = [C]^c[D]^d / [A]^a[B]^b |
| Reaction Rate | Rate = k[A]^m[B]^n |

🚀 Note: Remember that practice is key. The more you use these formulas, the more comfortable you will become with them.
3. Visualize the Reactions

Chemical reactions are not just about numbers; they involve real molecular interactions. Here’s how to visualize:
- Reaction Energy Diagrams: Sketch out energy diagrams for exothermic and endothermic processes. This helps understand energy changes visually.
- Equilibrium Shift: Use Le Chatelier’s principle to predict how changes in concentration, pressure, or temperature affect equilibrium.
- Molecular Modeling: Imagine how molecules might interact or change during a reaction.
This approach not only enhances understanding but also makes the subject more interesting and tangible.
4. Practice Problem-Solving

Worksheets like Chemistry Unit 4 Worksheet 2 are filled with problems that require critical thinking:
- Study Past Papers: Work through previous years’ exam questions to understand the types of problems likely to appear.
- Group Study: Collaborate with peers to solve problems collectively, discussing approaches and strategies.
- Timely Revision: Regularly revisit worksheets, textbook examples, and any notes to keep concepts fresh.
By practicing, you’ll become familiar with the types of questions and how best to approach them, reducing your test-day anxiety.
5. Seek Help When Needed

Don’t be afraid to ask for help:
- Tutors: If certain concepts remain elusive, consider hiring a tutor or using online tutoring platforms.
- Teachers: Utilize the expertise of your chemistry teacher. They can clarify doubts, provide tips, and correct misconceptions.
- Online Resources: Websites like Khan Academy, ChemGuide, and educational YouTube channels can offer additional explanations.
The key is to understand rather than memorize, which might require seeking different perspectives or explanations.
In wrapping up these tips for acing Chemistry Unit 4 Worksheet 2, remember that mastery comes from consistent practice and deep understanding. Leverage these tips to build confidence in your ability to handle thermochemistry, equilibrium, and all the related problems. Embrace the challenge, and you'll find that with the right strategies, chemistry can be not only conquerable but also enjoyable.
How can I remember all the chemical formulas?

+
One effective way to remember chemical formulas is through association. Link the formulas to real-life examples or visual aids. Additionally, using flashcards, apps, or games designed for memorization can also be helpful.
What is the best way to study for a chemistry exam?
+
The best study strategy includes a mix of conceptual understanding, regular practice with problem sets, group study sessions, and periodic revision. Also, teaching concepts to others can solidify your own understanding.
How important is understanding chemical equilibrium in daily life?

+
Equilibrium is crucial in understanding many industrial processes like fertilizer production, drug manufacturing, and even how our body maintains homeostasis. It’s all about balance and change in response to external conditions.