Chemistry Specific Heat Worksheet: Master Your Skills Easily
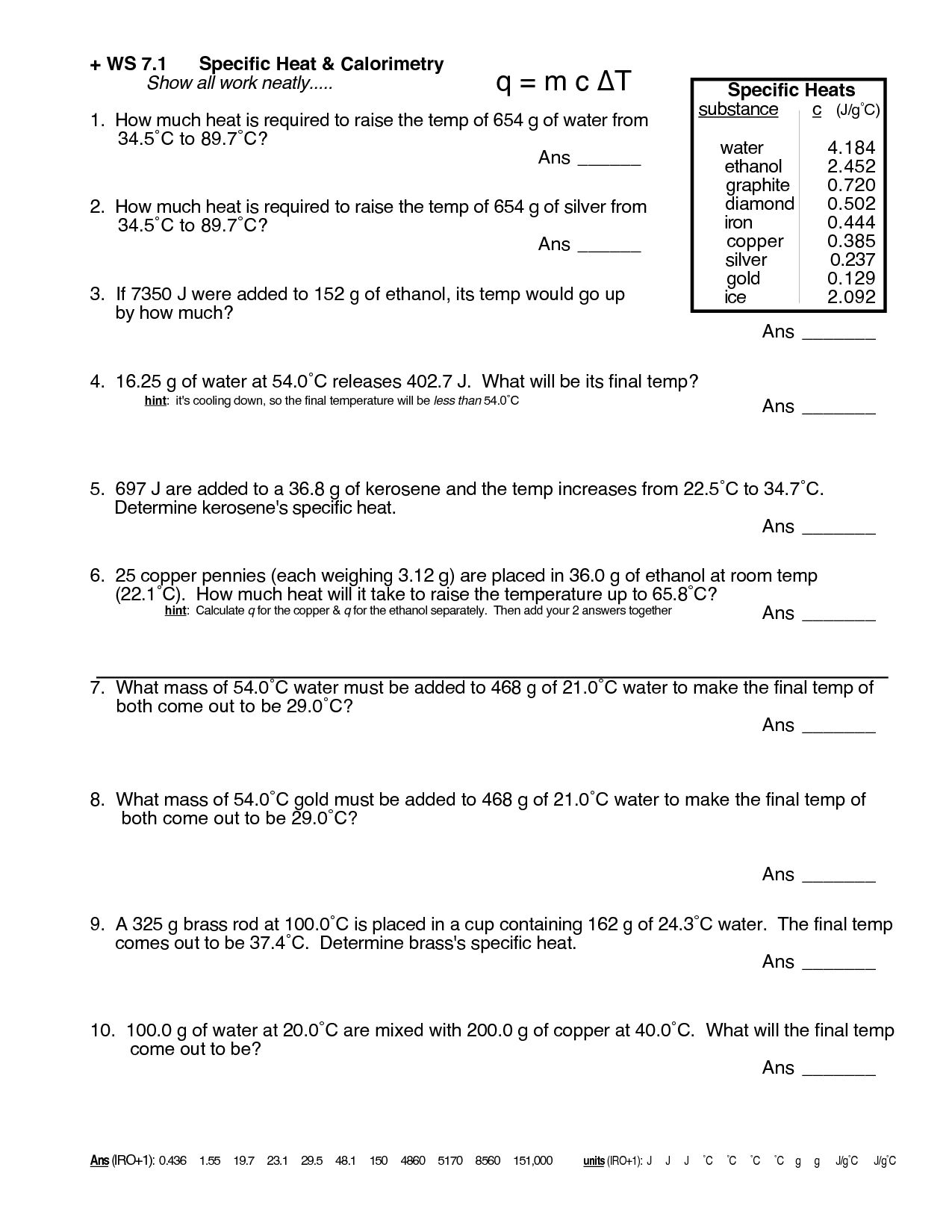
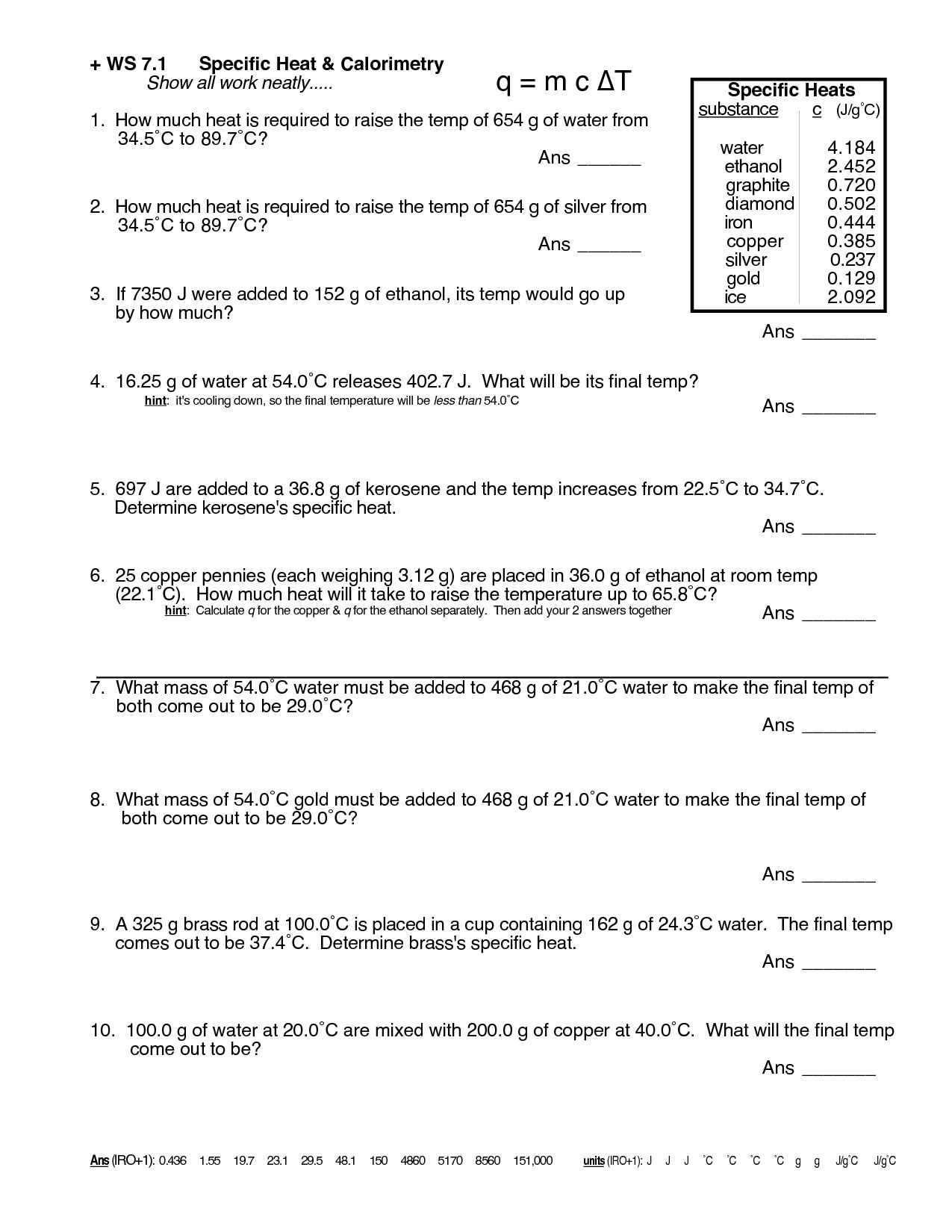
Exploring the depths of chemistry can be a fascinating journey, one filled with various phenomena, theories, and practical applications. One crucial concept that every chemistry student encounters is specific heat. Understanding specific heat not only helps in academic settings but is also fundamental for practical applications in fields like thermodynamics, material science, and engineering. Today, we'll delve into a detailed Chemistry Specific Heat Worksheet designed to enhance your understanding and mastery of this essential concept.
What is Specific Heat?

Specific heat, or specific heat capacity, is the amount of heat per unit mass required to raise the temperature of a substance by one degree Celsius. It's typically measured in Joules per gram degree Celsius (J/g°C). This property varies from one substance to another, reflecting how much energy is needed to change the internal energy of the substance without altering its phase.
The Chemistry Specific Heat Worksheet

To truly grasp specific heat, working through practical problems is highly beneficial. Here's a structured worksheet to guide you:
Section 1: Definitions and Principles

- Define Specific Heat: Write down the definition of specific heat in your own words.
- Heat Capacity vs. Specific Heat: Explain the difference between these two concepts.
- Application: Describe one real-life scenario where understanding specific heat could be useful.
Section 2: Calculations

Calculate the heat absorbed or released in the following problems:
| Problem | Mass (g) | Temperature Change (°C) | Specific Heat (J/g°C) |
|---|---|---|---|
| 1 | 20.0 | 30.0 | 4.18 |
| 2 | 50.0 | 100.0 | 0.90 |
| 3 | 75.0 | 25.0 | 2.46 |

🔍 Note: Use the formula Q = mc\Delta T to solve these problems.
Section 3: Comparative Analysis

- Compare the specific heat of water to that of copper. What does this indicate about their thermal properties?
- How would you explain to a layperson why water is often used in heating systems?
Section 4: Experimental Setup

Propose an experiment to determine the specific heat of an unknown metal. Outline the following:
- Materials needed
- Procedure
- Variables controlled and measured
- How to analyze the data
Through this worksheet, not only will you understand the concept of specific heat, but you'll also apply it in various contexts, enhancing your problem-solving skills in chemistry.
Key Takeaways from the Worksheet

- The relationship between mass, temperature change, and heat energy.
- The significance of specific heat in thermal energy transfers.
- How different materials absorb and release heat differently due to their atomic structure and bonding.
By completing this worksheet, you solidify your understanding of specific heat, making you better prepared for further studies or practical applications in real-world scenarios.
Why Master Specific Heat?
Mastering the concept of specific heat can have broad implications: - Energy Efficiency: Understanding how materials respond to heat helps in designing more efficient heating and cooling systems. - Material Science: It’s pivotal in selecting materials for specific applications, like insulation or heat sinks. - Chemical Reactions: Specific heat affects the thermodynamics of reactions, particularly in industrial processes.
As we move away from the worksheet and delve into broader applications, remember that the principles of specific heat are foundational not only in chemistry but in our everyday lives, from cooking to industrial processes.
What is the difference between heat capacity and specific heat?

+
Heat capacity is the amount of heat needed to change the temperature of an object by one degree Celsius or Kelvin. Specific heat, on the other hand, is the amount of heat per unit mass required to do the same, making it an intensive property, whereas heat capacity is extensive.
Can specific heat change with temperature?

+
Yes, specific heat can change with temperature due to different modes of energy storage in the material changing with temperature. However, for many practical purposes, it is often treated as constant over small temperature ranges.
How do I use specific heat in a lab?

+
In a lab, you might use specific heat to calculate the amount of heat absorbed or released by a substance when its temperature changes. This could be part of calorimetry experiments or material analysis.