5 Tips for Mastering Chemistry Significant Figures

When it comes to chemistry, mastering the art of significant figures (sig figs) is crucial. Significant figures are a means to represent the precision of measurements, and understanding how to correctly handle them can mean the difference between a perfect experiment and an imprecise result. Here are five tips to get you on your way to becoming a significant figures expert.
Understand the Rules

Before you dive into calculations, you need to know the fundamental rules:
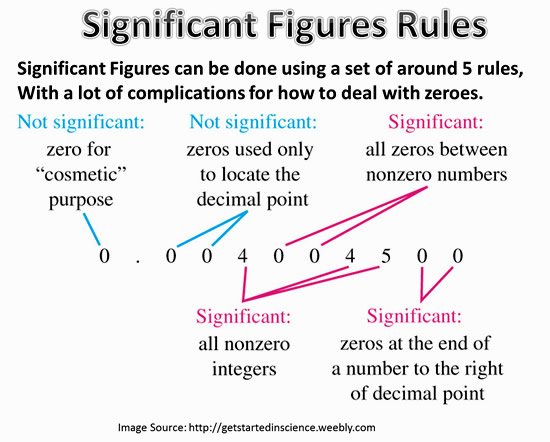
- Non-zero digits are always significant.
- Zeros between non-zero digits are significant.
- Leading zeros are never significant.
- Trailing zeros are significant if there is a decimal point; otherwise, they could be significant or not, depending on the context.
- Exact numbers (defined numbers) have an infinite number of significant figures.
🔍 Note: Remember that context matters. Always consider the origin of the number for proper handling.
Practice with Examples

The best way to internalize these rules is through practice:
- Count sig figs: For instance, in the number 0.00305, there are 3 significant figures. Here’s the breakdown:
- 0.00 (leading zeros, not significant)
- 3 (non-zero, significant)
- 0 (trailing zero after a decimal point, significant)
- 5 (non-zero, significant)
- Perform Arithmetic: When you multiply or divide, the answer should be rounded to match the measurement with the least number of significant figures. In addition or subtraction, the answer must have the same number of decimal places as the number with the least decimal places.
Use a Sig Fig Calculator

To avoid human error, consider using a significant figures calculator. Here’s a basic guide on how to use one:
| Step | Action |
|---|---|
| 1 | Input the number you want to evaluate |
| 2 | Select the operation (if applicable) |
| 3 | Click calculate to see the result |

💡 Note: While calculators are useful, they can’t replace understanding the rules. Use them to verify your manual calculations.
When to Round

Rounding isn’t just a math class exercise; it’s integral to chemistry:
- Round after all calculations to avoid cumulative errors.
- Use the rounding rule: if the digit to be dropped is 5 or more, the preceding digit is increased by one.
- Be aware that different scenarios require different rounding strategies.
For example, when dividing 5.48 by 2.01 (which has three and two sig figs respectively), the result is 2.726368159, which rounds to 2.7 according to the least number of significant figures rule.
Why They Matter

Understanding why significant figures are essential can make learning them easier:
- Accuracy: Sig figs reflect the level of precision in your measurements.
- Communication: They tell others the level of detail in your results.
- Precision: They help maintain accuracy throughout calculations.
Mastering significant figures also helps in understanding measurement errors, and their effect on the reliability of your experiments.
Mastering significant figures is like learning the grammar of the language of chemistry. Just as good grammar ensures clear communication in writing, proper use of significant figures ensures precision and accuracy in science. From the basic rules to practical applications, these tips will help you approach chemistry with confidence. Remember, it's not just about getting the right answer, but about understanding why your calculations work the way they do. With practice, using tools effectively, and recognizing the importance of significant figures, you'll navigate the complexities of chemistry with ease and precision.
What are significant figures?
+
Significant figures, often abbreviated as sig figs, are the digits of a number that carry meaning contributing to its precision. They are used to communicate the level of accuracy of a measurement.
Why do chemists use significant figures?

+
Chemists use significant figures to maintain accuracy when recording and reporting measurements. They help convey the precision of those measurements and are critical for preventing the propagation of rounding errors during calculations.
Can the number of significant figures change?

+
Yes, the number of significant figures can change depending on the context, like during addition, subtraction, multiplication, or division. The answer should have as many sig figs as the measurement with the least number of sig figs involved in the calculation.