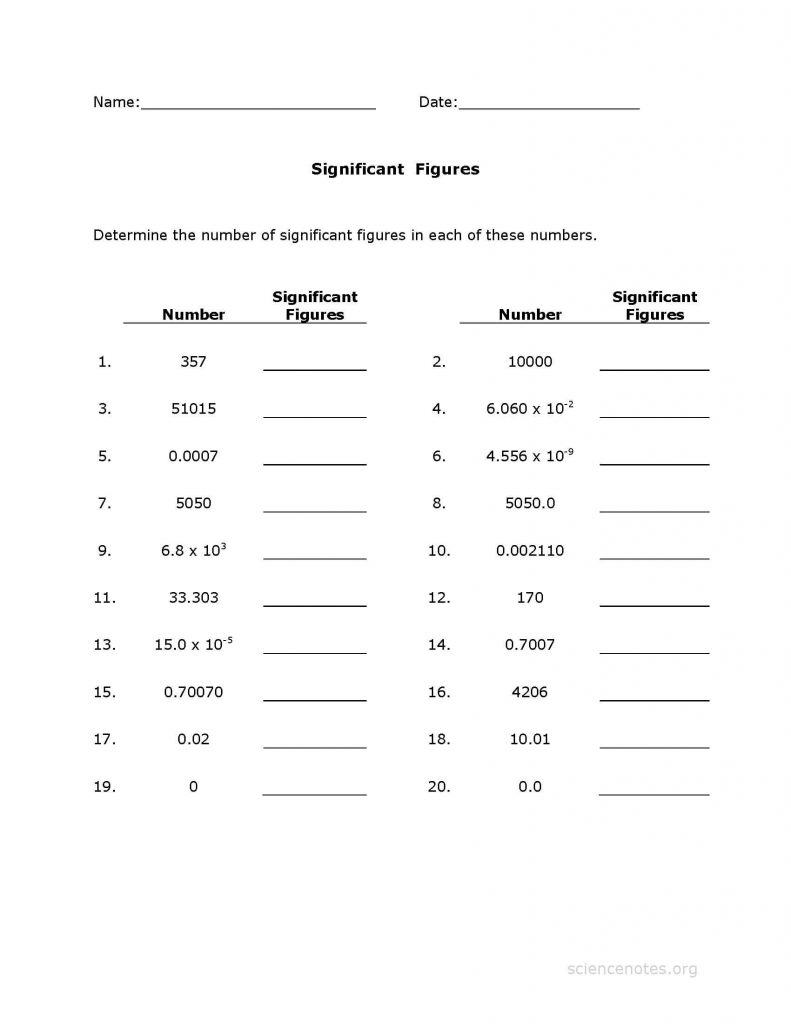
5 Essential Tips for Solving Chemistry Significant Digits Worksheets

Significant digits, often referred to as significant figures, play a critical role in chemistry for representing and calculating measurements with precision. Whether you're a student tackling a worksheet or a professional dealing with complex calculations, understanding significant digits is fundamental to ensure accuracy and maintain scientific integrity. Here are five essential tips to help you master these worksheets effectively:
1. Understand the Rules of Significant Figures


Before diving into any worksheet, you need to know the basic rules for determining significant figures:
- Non-zero digits are always significant.
- Zeros between non-zero digits are significant (e.g., 505 has three significant figures).
- Leading zeros in a number less than one are not significant (e.g., 0.003 has one significant figure).
- Trailing zeros in numbers with a decimal point are significant (e.g., 3.000 has four significant figures).
- Exact numbers, derived from counting or definitions, have an infinite number of significant figures.
Understanding these rules allows you to identify significant digits quickly, which is crucial when you are dealing with various forms of data in your worksheets.
2. Practice Precision and Accuracy
When dealing with measurements, precision and accuracy are distinct but related concepts:
- Precision refers to how close repeated measurements are to each other. This influences the number of significant digits you report.
- Accuracy measures how close a measurement is to the true value.
Remember, when adding or subtracting, your answer should only reflect the least precise value involved in the calculation. For multiplication and division, the result should have no more significant digits than the least significant number in your calculations. Here’s an example:
| Calculation | Significant Figures in Each Number | Rule Applied | Result (Significant Figures) |
|---|---|---|---|
| 150.0 + 25.3 | 4, 3 | Addition/Subtraction | 175.3 (3 significant figures) |
| 5.00 * 1.05 | 3, 3 | Multiplication/Division | 5.25 (3 significant figures) |

🔬 Note: Calculations require rounding, so understanding the rules of significant figures can help maintain precision and accuracy.
3. Use Digital Tools Wisely

While calculators and software can perform operations with high precision, remember that they might not automatically handle significant digits correctly:
- Always round your final answer to the correct number of significant figures manually.
- Understand the limitations of your tools; many calculators will provide too many decimal places for a scientifically accurate result.
- Use tools like scientific calculators or online significant figure calculators that allow you to specify the number of significant figures you want to keep.
4. Master the Art of Rounding

When working through a worksheet:
- Learn to round numbers according to the rules of significant figures. If the digit following the last significant digit is 5 or more, round up; if it’s less than 5, round down.
- Practice this skill frequently. Rounding incorrectly can lead to errors in subsequent calculations.
- Remember that rounding should be done at the end of a set of calculations, not after each individual step, to minimize the accumulation of errors.
5. Apply Knowledge to Real-World Scenarios

Chemistry isn’t just about calculations on paper. To truly understand significant figures:
- Consider how measurements are taken in a lab setting. Instruments have different levels of precision, which affects how many significant figures you should report.
- Think about reporting results in a lab report or a publication. Over-reporting significant figures can misleadingly suggest a higher level of precision than actually exists.
- Use case studies or examples from real-world applications to solidify your understanding. For instance, when calculating the concentration of a chemical in a solution, the number of significant figures should reflect the accuracy of the measurement tools used.
Significant figures are not just a part of the curriculum but a key aspect of scientific communication. They ensure that precision is communicated accurately between scientists, thereby maintaining the credibility of research and data. The rules and principles of significant figures allow us to represent measurements in a way that reflects their true precision, avoiding both under-reporting and over-reporting of data. By following these tips, you'll not only improve your performance on chemistry worksheets but also sharpen your scientific literacy, making you a more competent and reliable chemist.
Why do significant figures matter in chemistry?

+
Significant figures help communicate the precision of measurements. They indicate the reliability and accuracy of data in scientific work, ensuring that results can be trusted and compared correctly among different researchers.
Can significant figures affect the outcome of experiments?

+
Absolutely. Incorrect handling of significant figures can lead to results that appear more or less precise than they truly are, which can skew interpretations and conclusions, possibly invalidating experimental outcomes.
How do you handle trailing zeros?

+
Trailing zeros after a decimal point are significant. If a number like 23.000 is given, all five digits are significant. However, trailing zeros before a decimal point, as in 2300, are not significant unless specified otherwise or if there’s a decimal point following them, like in 2300.0.