Master Your Chemistry Skills: Reaction Worksheet Guide

Delving into the world of chemistry, one quickly discovers that reactions are the fundamental processes that transform substances. Understanding these reactions is not just a matter of academic interest but a practical skill necessary for fields ranging from pharmaceuticals to environmental science. This comprehensive guide aims to walk you through a structured approach to mastering chemistry reactions with a detailed worksheet designed to help you solidify your understanding and enhance your skills.
Why Master Chemistry Reactions?


Chemistry reactions are not just equations; they are the core of chemical interactions. Here's why mastering them is crucial:
- Scientific Understanding: Learning reactions provides insight into how atoms rearrange to form new substances.
- Problem-Solving: It improves your ability to analyze complex problems and devise solutions.
- Application in Various Fields: From manufacturing processes to medicine, understanding reactions is vital for applied sciences.
Worksheet Guide: How to Learn and Apply Chemistry Reactions

1. Identify Reaction Types
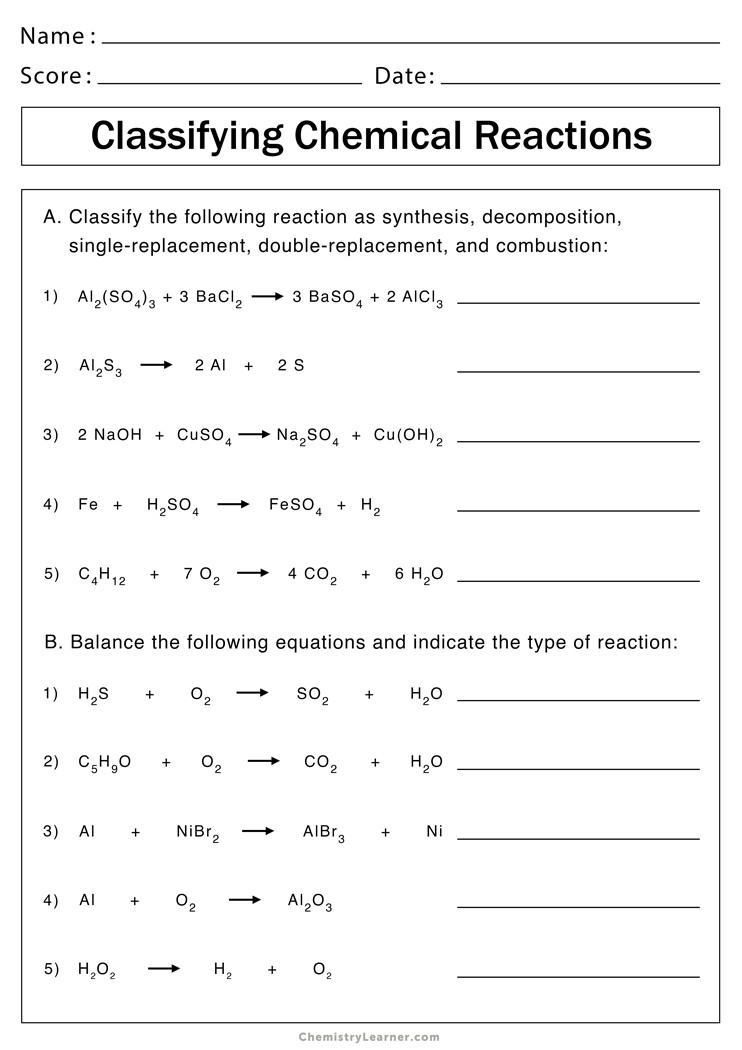
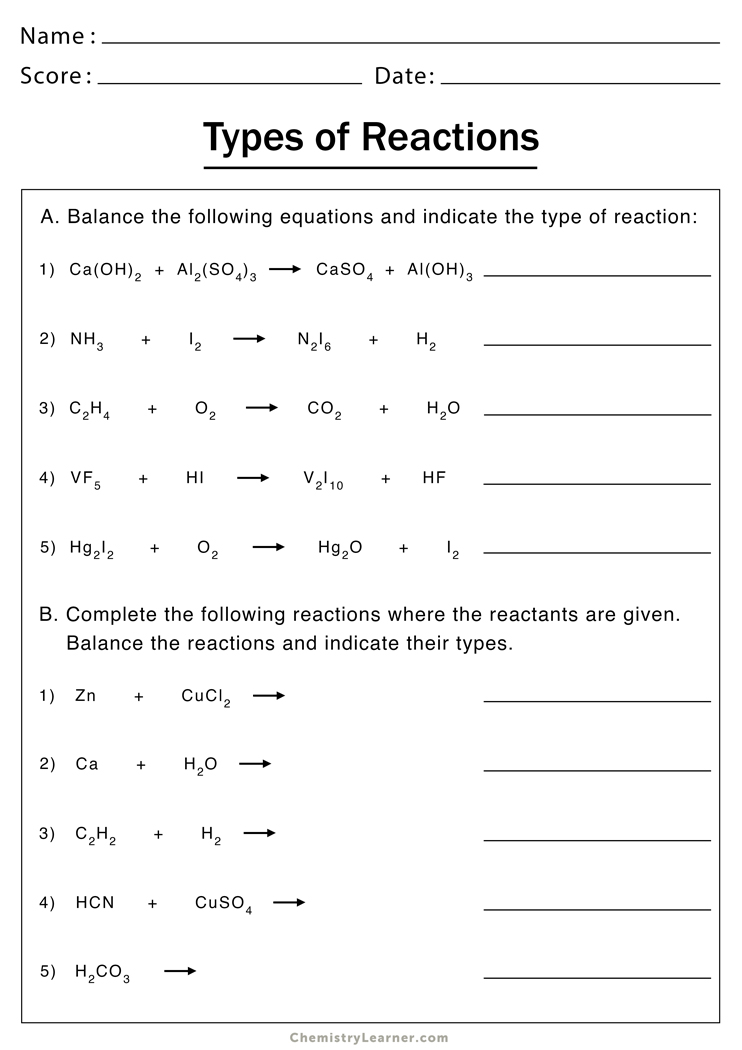
Start by familiarizing yourself with common types of reactions such as synthesis, decomposition, single displacement, double displacement, combustion, and acid-base reactions. Here’s a quick look at each:
| Type of Reaction | Description |
|---|---|
| Synthesis | Two or more substances combine to form a single compound. |
| Decomposition | A single compound breaks down into two or more simpler substances. |
| Single Displacement | One element replaces another in a compound. |
| Double Displacement | The anions and cations of two different compounds switch places. |
| Combustion | A substance reacts with oxygen, often releasing energy as heat or light. |
| Acid-Base | An acid reacts with a base to produce water and a salt. |

💡 Note: Identifying the reaction type helps predict the outcomes and balance the equations more efficiently.
2. Practice Balancing Equations

Balancing chemical equations is a skill you’ll use constantly in chemistry. Here’s a step-by-step guide:
- Write the unbalanced equation.
- Count the number of atoms for each element in both the reactants and products.
- Adjust coefficients to balance the number of atoms for each element.
- Check your work by verifying each element’s atoms balance.
3. Classify Reactions and Predict Products

With the reaction type identified, you can often predict the products:
- Double displacement reactions often form precipitates, gases, or water.
- Single displacement reactions use the activity series to determine if the reaction occurs.
4. Understand Energy Changes

Reactions can either release energy (exothermic) or absorb it (endothermic). Recognize:
- Exothermic reactions are characterized by a release of heat or light.
- Endothermic reactions absorb energy, often causing cooling or requiring heat input.
5. Apply Reaction Principles

Here’s where you put your knowledge to practice:
- Understand stoichiometry: Use balanced equations to calculate the quantity of reactants and products involved in reactions.
- Limiting reactants: Determine which reactant will be used up first.
- Reaction yield: Calculate the amount of product formed compared to the theoretical yield.
Recapitulating Key Points

In this guide, we’ve explored the necessity of mastering chemical reactions, provided a systematic approach to learn and apply these concepts through a comprehensive worksheet. The ability to identify, predict, balance, and understand the energy dynamics of reactions is not only essential for academic pursuits but also crucial for practical applications in various scientific disciplines. By internalizing these principles, you’re equipping yourself with a robust skill set that enables you to analyze, solve, and innovate within the realm of chemistry.
What are the most common types of chemical reactions?

+
The most common types of chemical reactions include synthesis, decomposition, single displacement, double displacement, combustion, and acid-base reactions.
How can one predict the products of a chemical reaction?
+
To predict products, identify the type of reaction and apply relevant principles. For instance, in a double displacement, use solubility rules to determine if a precipitate forms.
What are exothermic and endothermic reactions?

+
Exothermic reactions release energy, often as heat or light. Endothermic reactions absorb energy from their surroundings, which can cause cooling or require an energy input to proceed.